Table of contents
Selected papers
October 2025
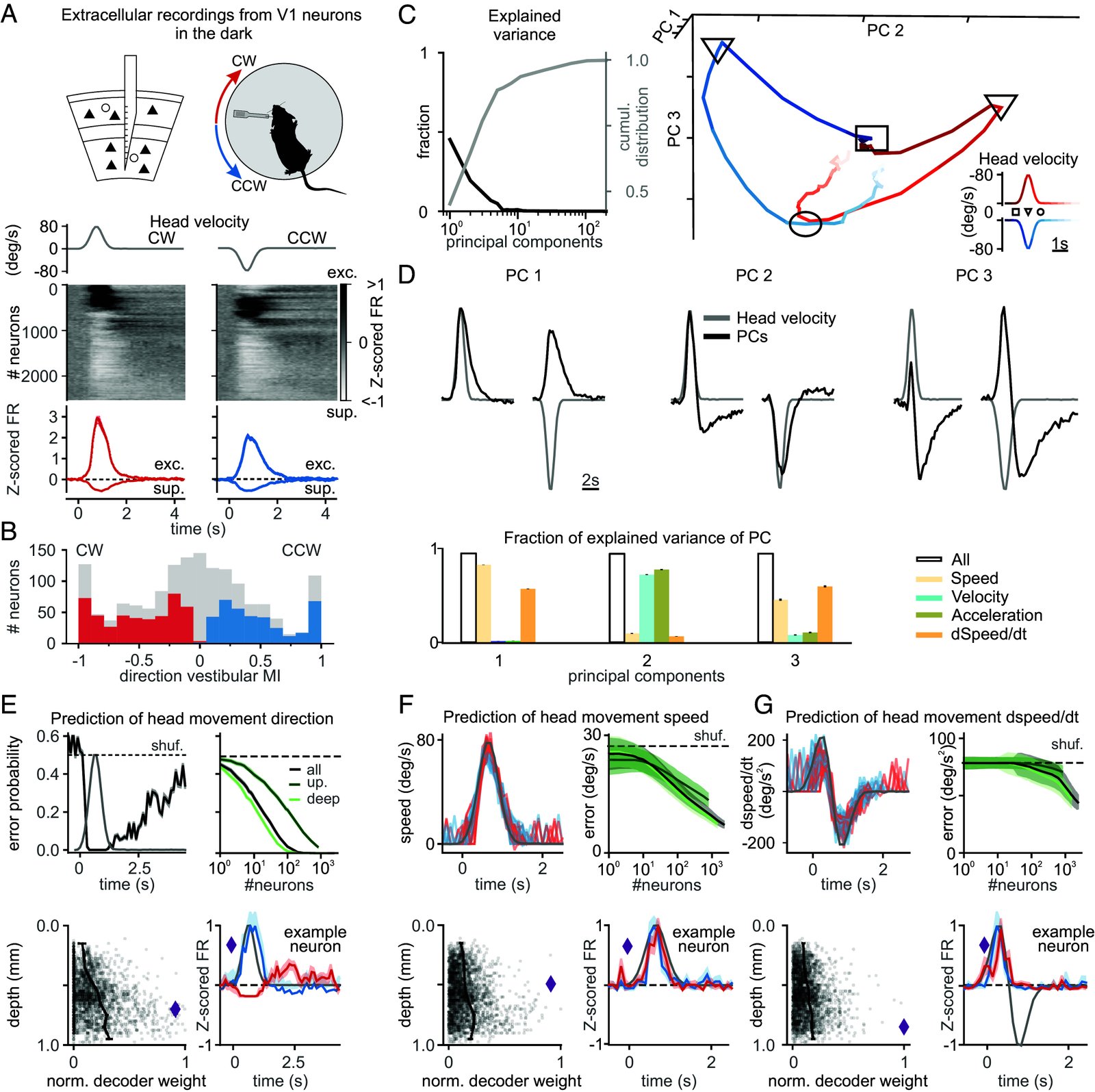
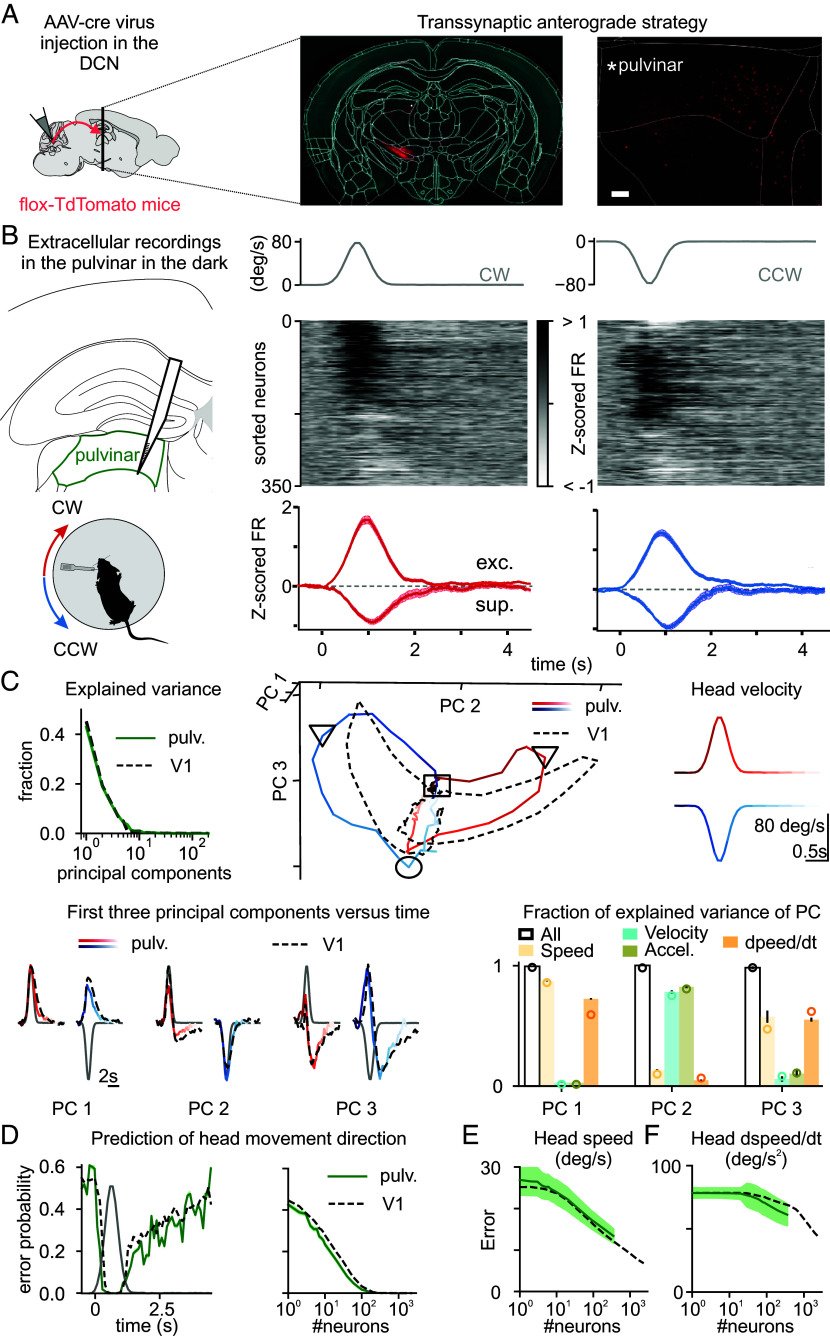
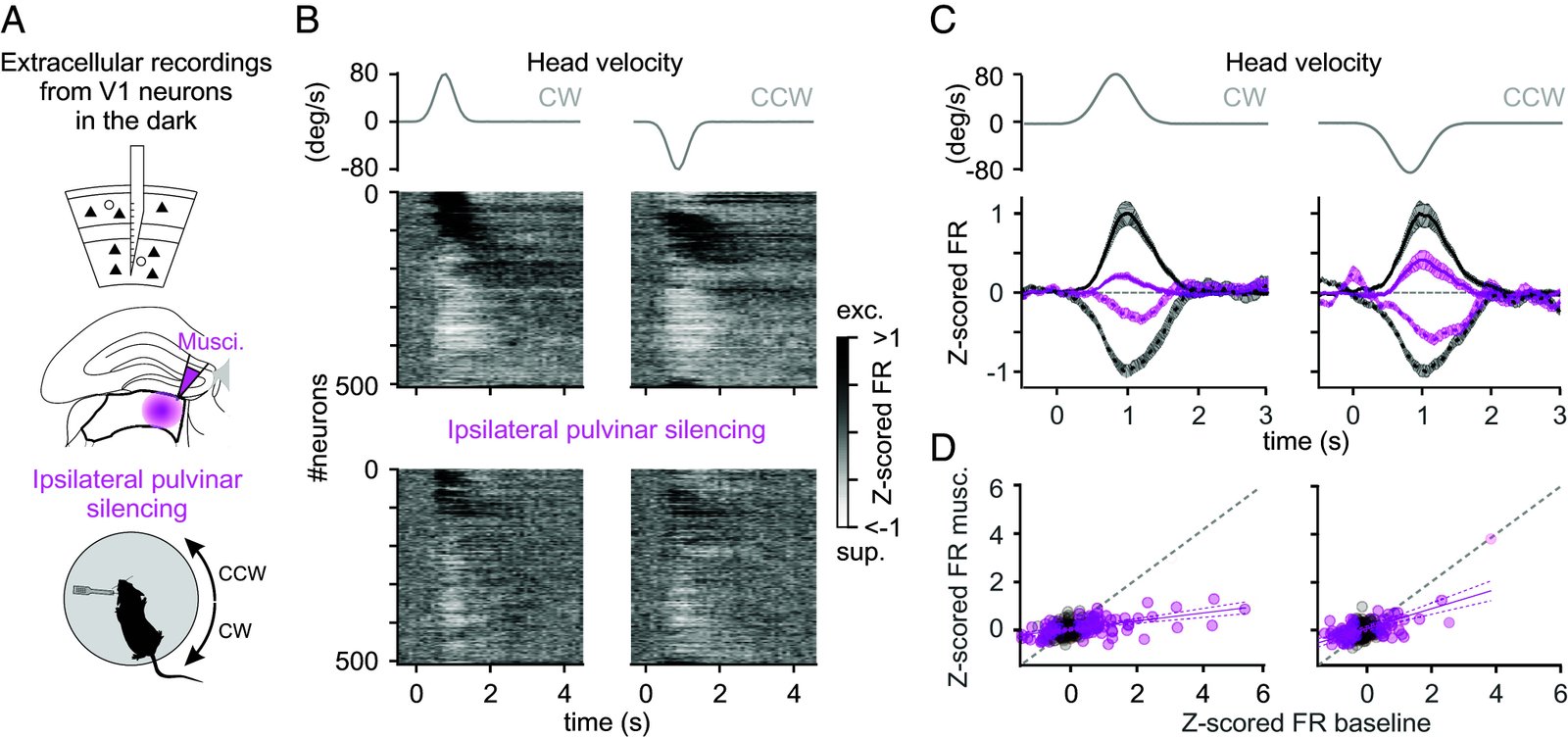
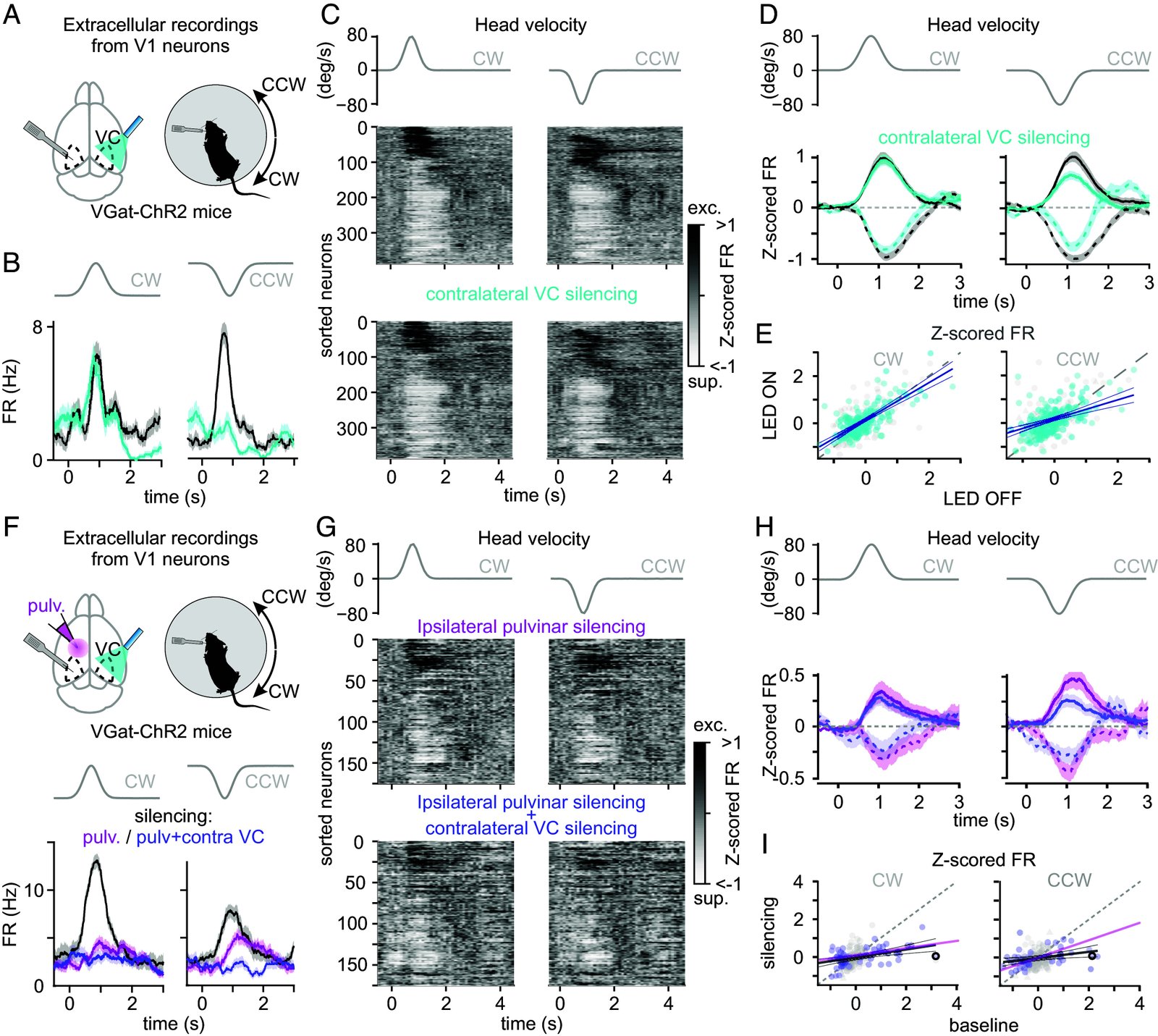
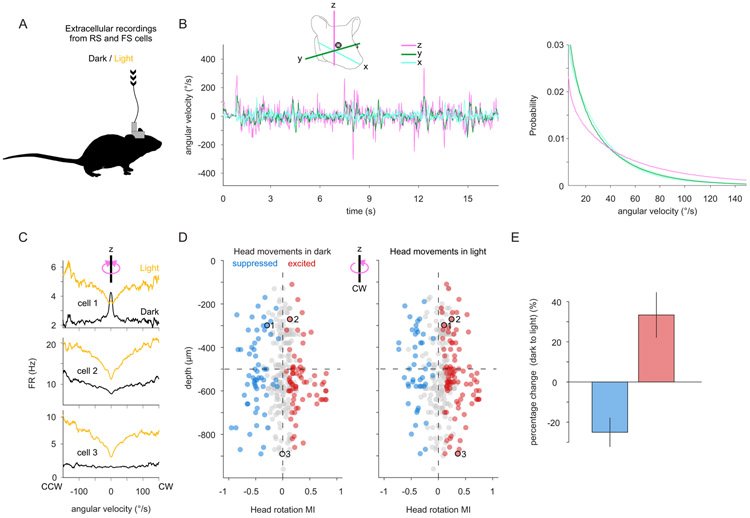
Head movements are sensed by the vestibular organs. Unlike classical senses, signals from vestibular organs are not selectively conveyed to a dedicated cortical area but are broadcast throughout the cortex. This distributed processing pattern reflects the fundamental role of vestibular information in contextual modulation across diverse cortical computations. Surprisingly, the routes taken by vestibular signals to reach the cortex are still largely uncharted. Here, we show that the primary visual cortex (V1) receives real-time head movement signals-direction, velocity, and acceleration-from the ipsilateral pulvinar and contralateral visual cortex (VC). The ipsilateral pulvinar provides the main head movement signal, with a bias toward contraversive movements (e.g., clockwise movements in left V1). Conversely, the contralateral VC provides head movement signals during ipsiversive movements. Crucially, head movement variables encoded in V1 are already encoded in the pulvinar, suggesting that those variables are computed subcortically. Thus, the convergence of inter- and intrahemispheric signals endows V1 with a rich representation of the animal's head movements.
May 2024
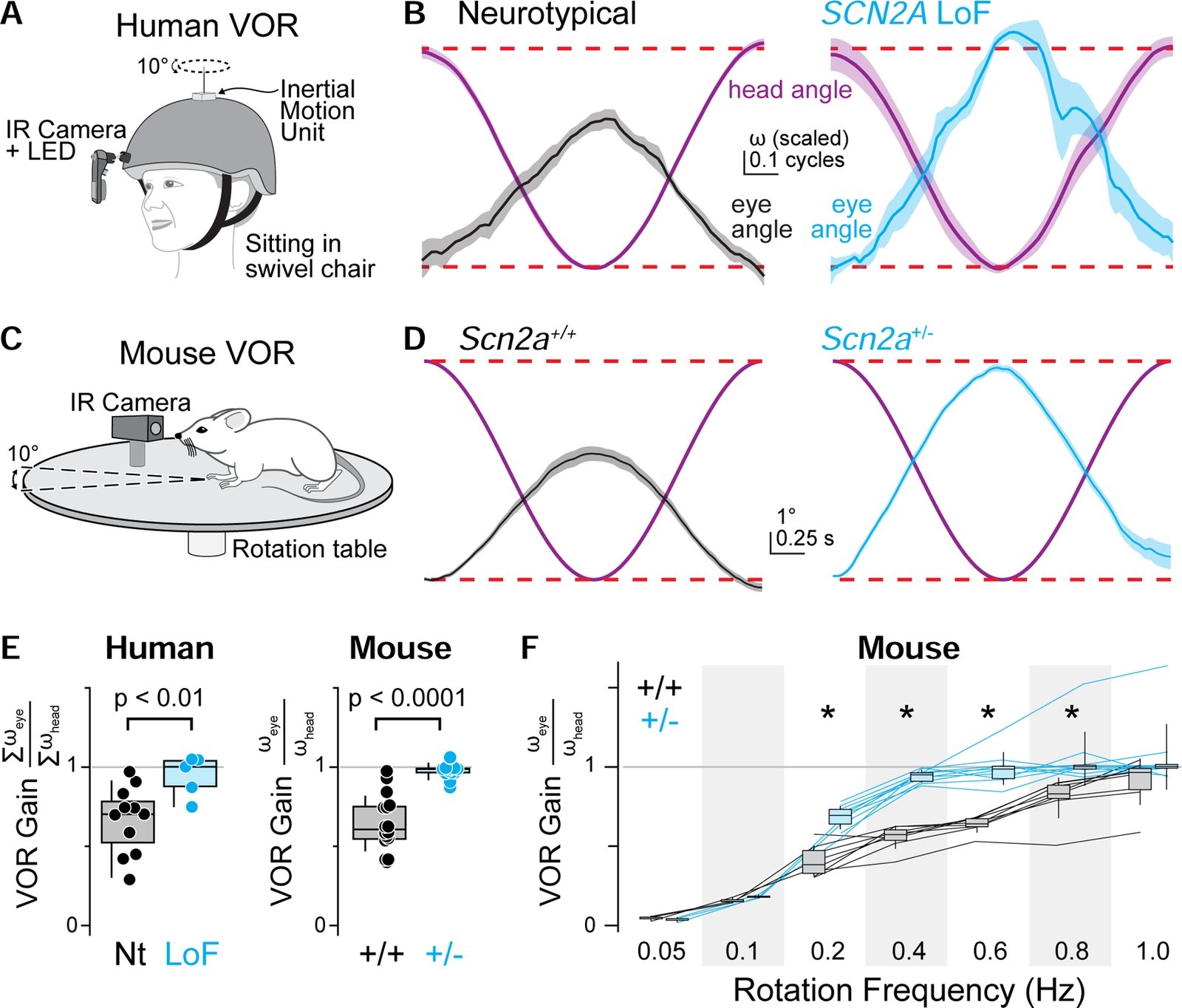
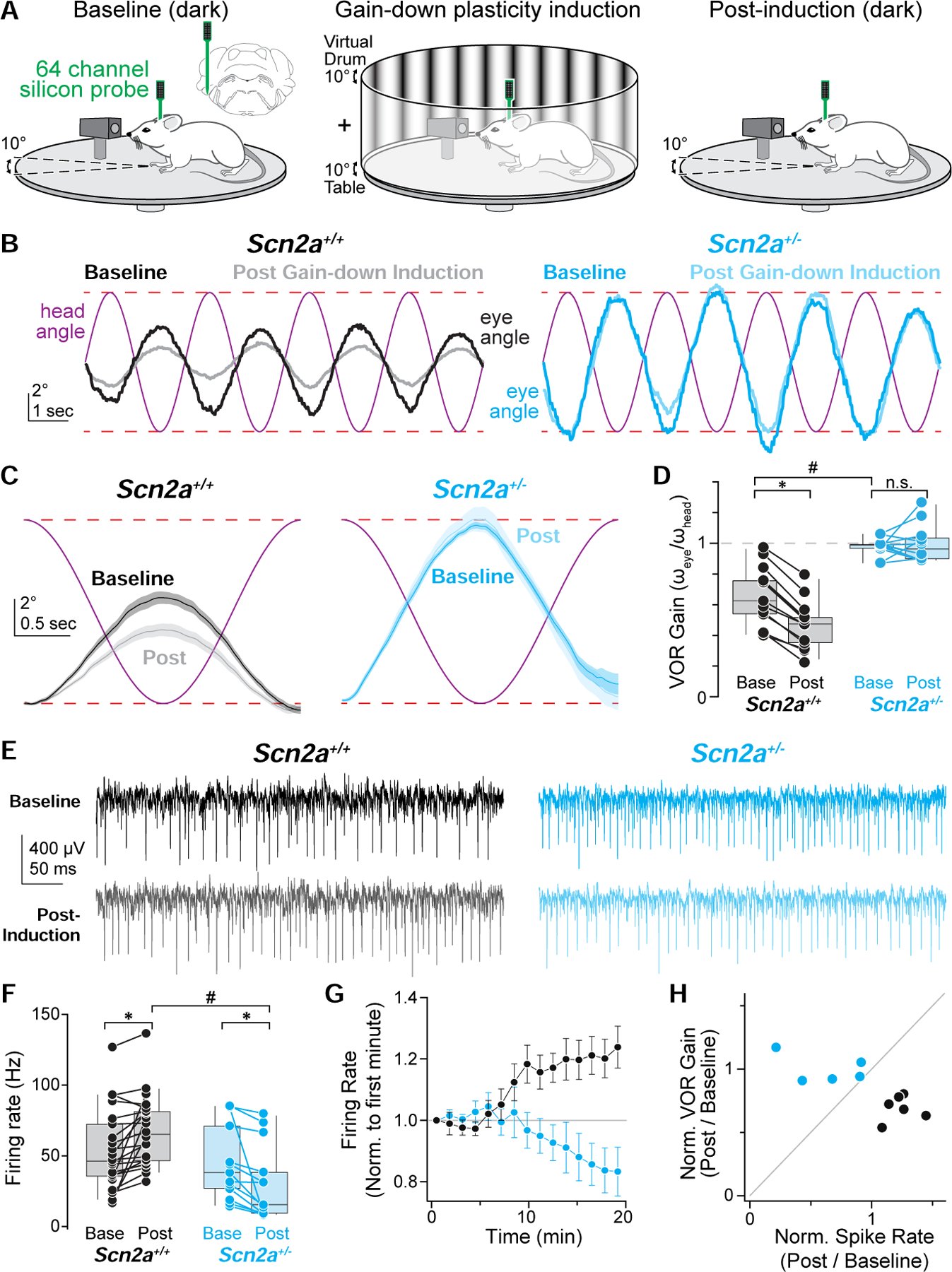
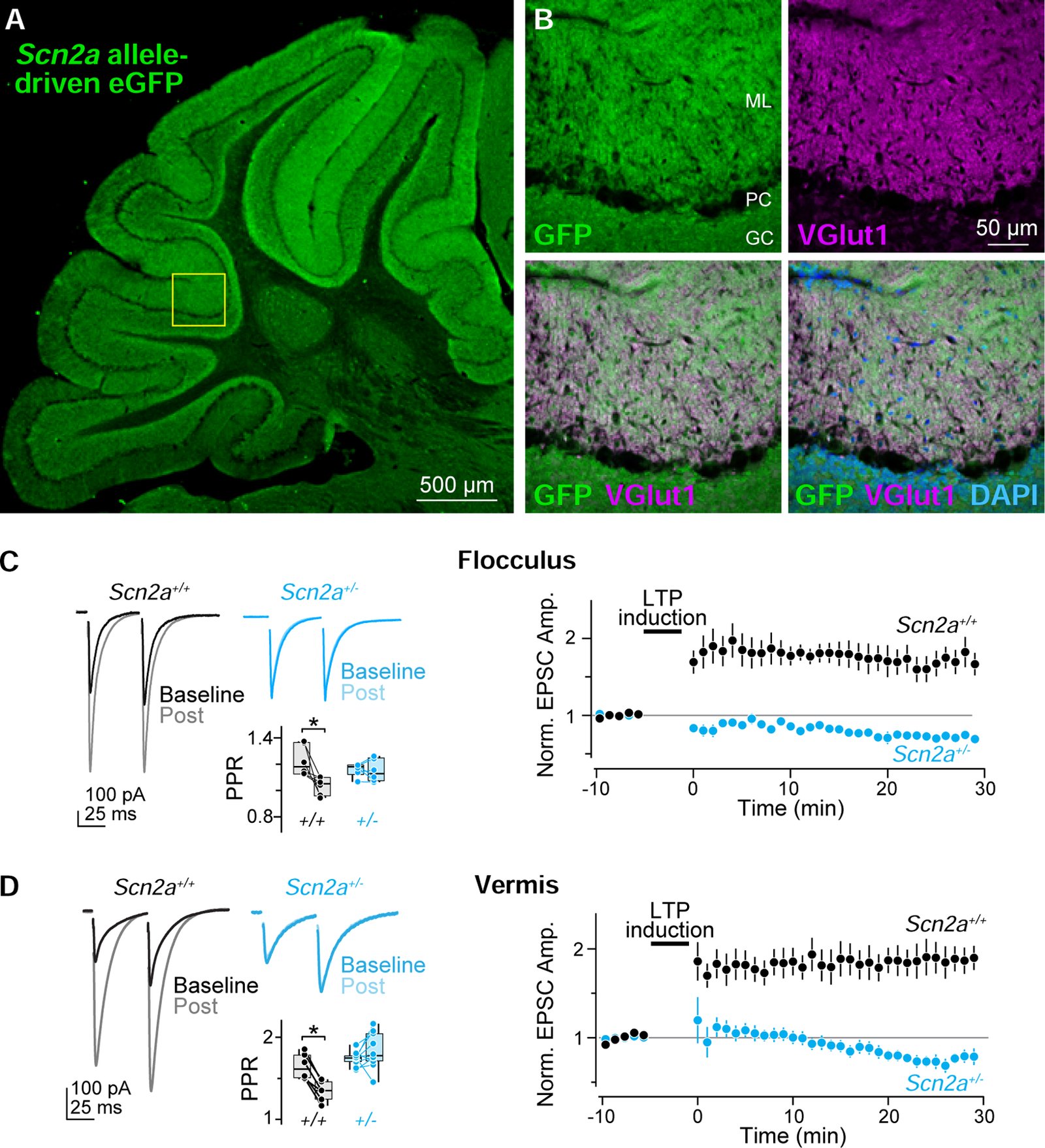
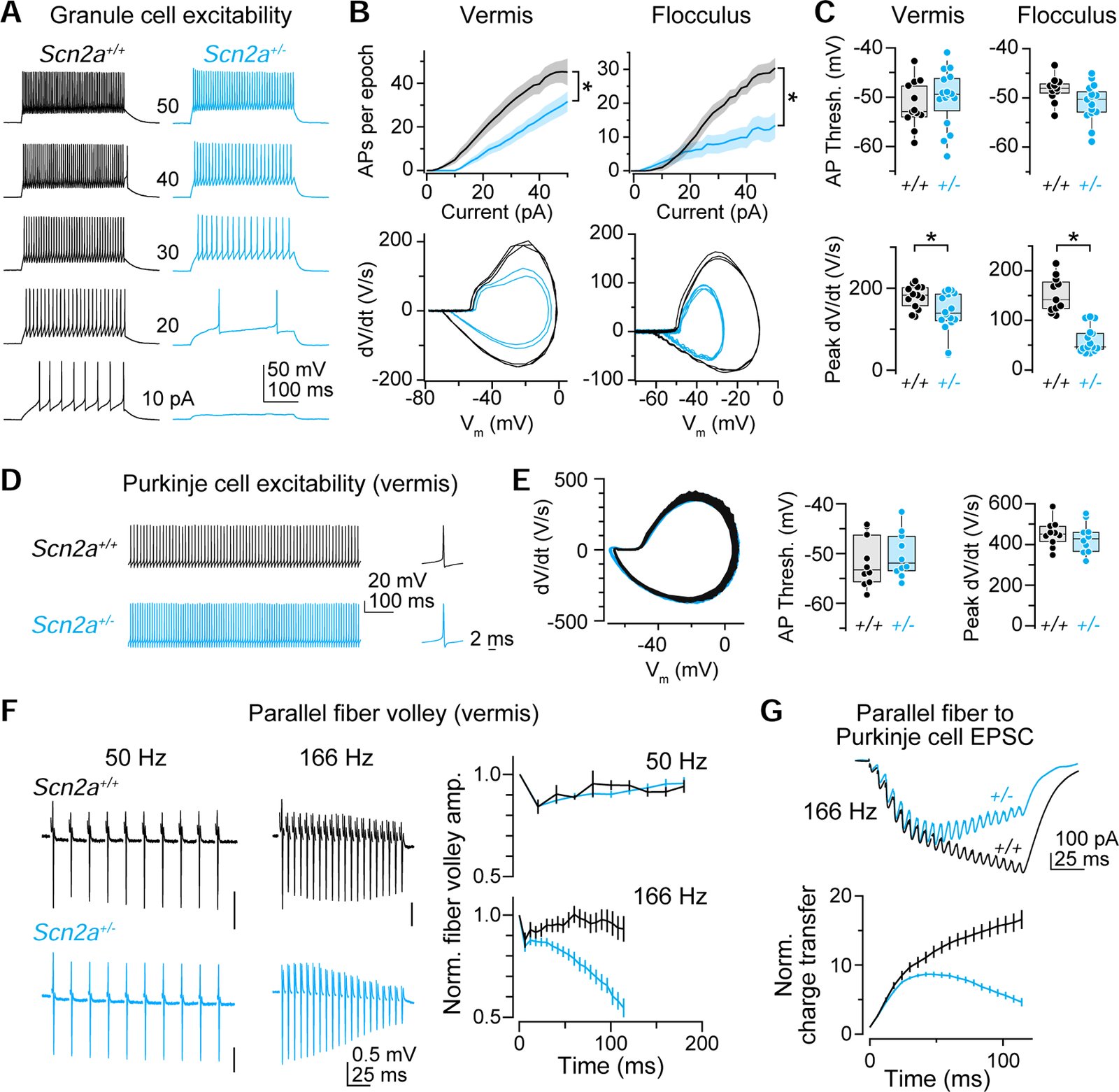
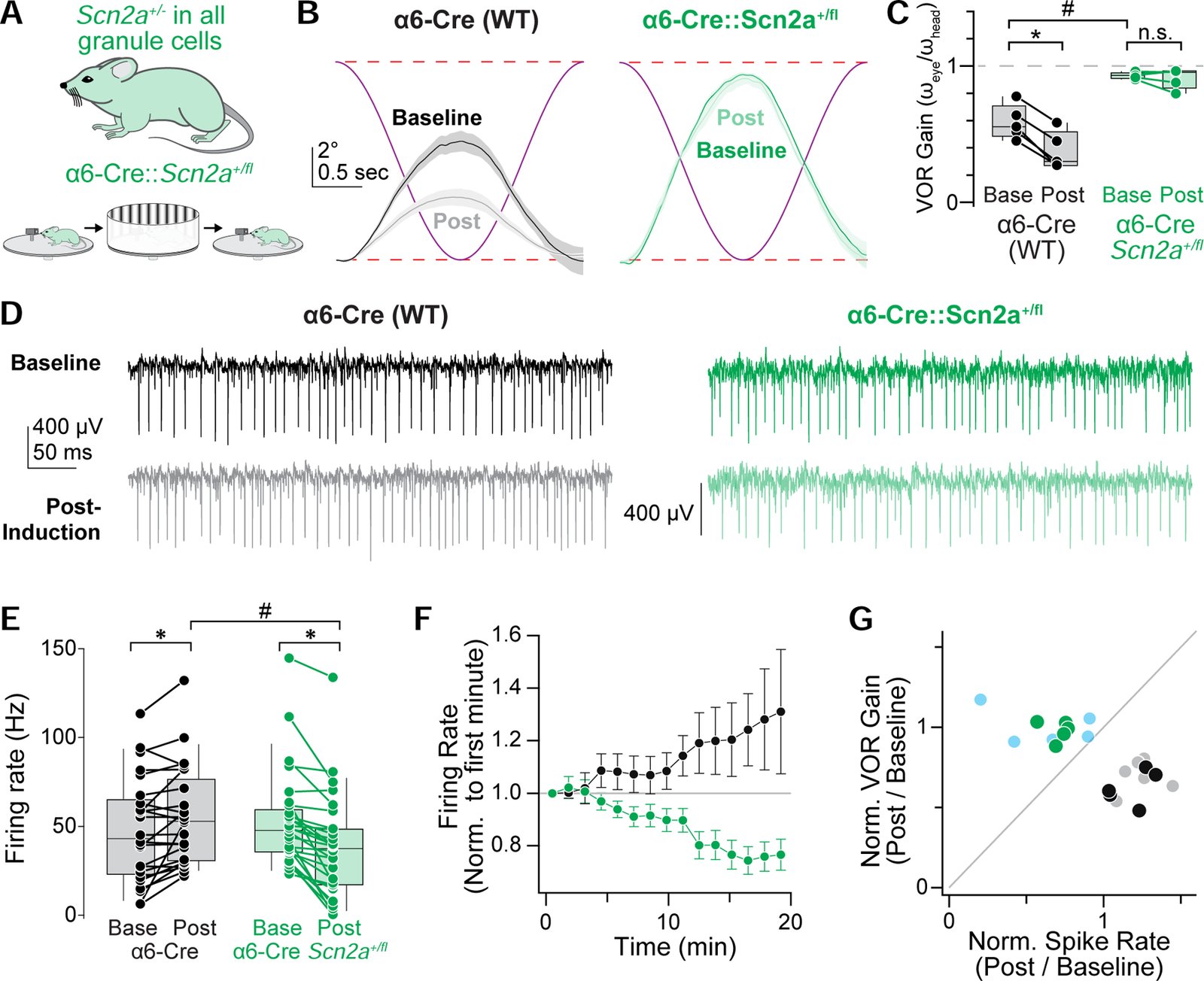
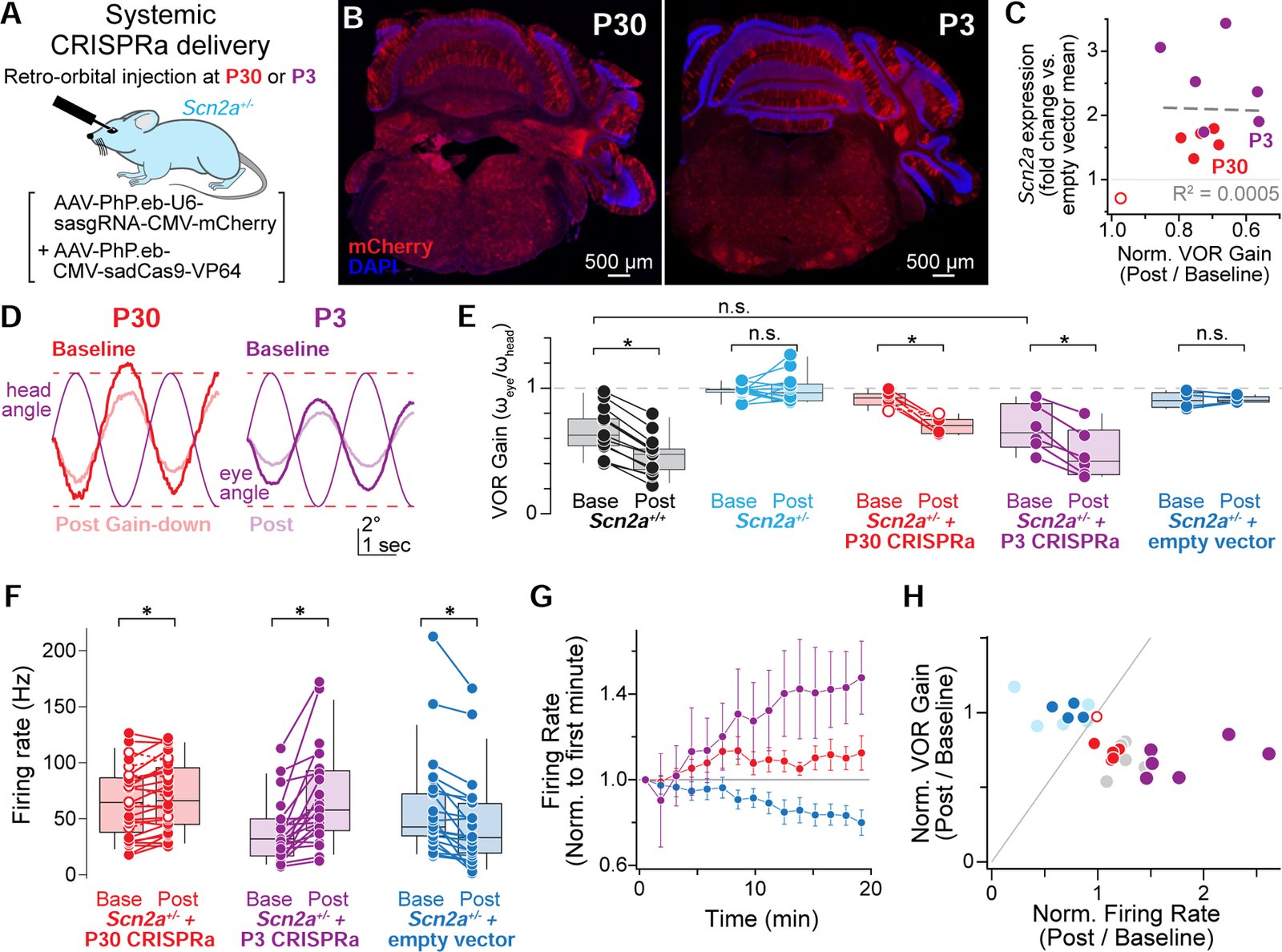
Children diagnosed with autism spectrum disorder (ASD) commonly present with sensory hypersensitivity or abnormally strong reactions to sensory stimuli. Such hypersensitivity can be overwhelming, causing high levels of distress that contribute markedly to the negative aspects of the disorder. Here, we identify a mechanism that underlies hypersensitivity in a sensorimotor reflex found to be altered in humans and in mice with loss of function in the ASD risk-factor gene SCN2A. The cerebellum-dependent vestibulo-ocular reflex (VOR), which helps maintain one's gaze during movement, was hypersensitized due to deficits in cerebellar synaptic plasticity. Heterozygous loss of SCN2A-encoded NaV1.2 sodium channels in granule cells impaired high-frequency transmission to Purkinje cells and long-term potentiation, a form of synaptic plasticity important for modulating VOR gain. VOR plasticity could be rescued in mice via a CRISPR-activator approach that increases Scn2a expression, demonstrating that evaluation of a simple reflex can be used to assess and quantify successful therapeutic intervention.
December 2023
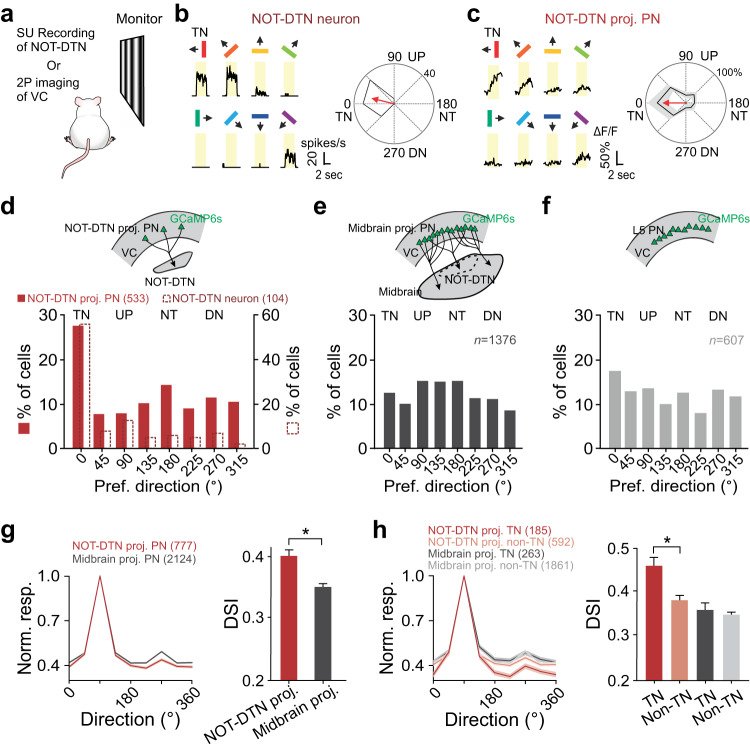
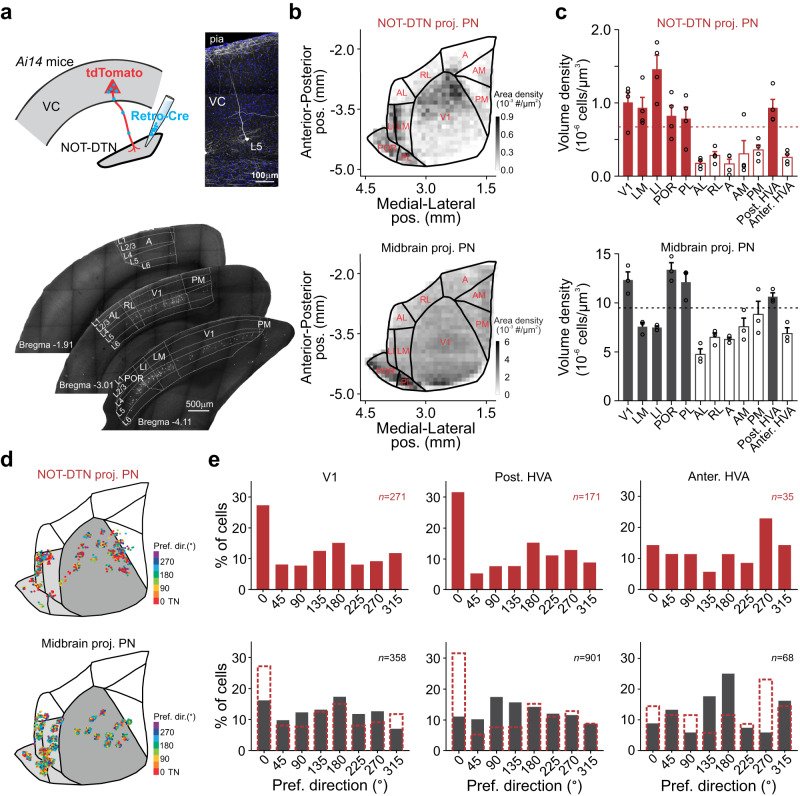
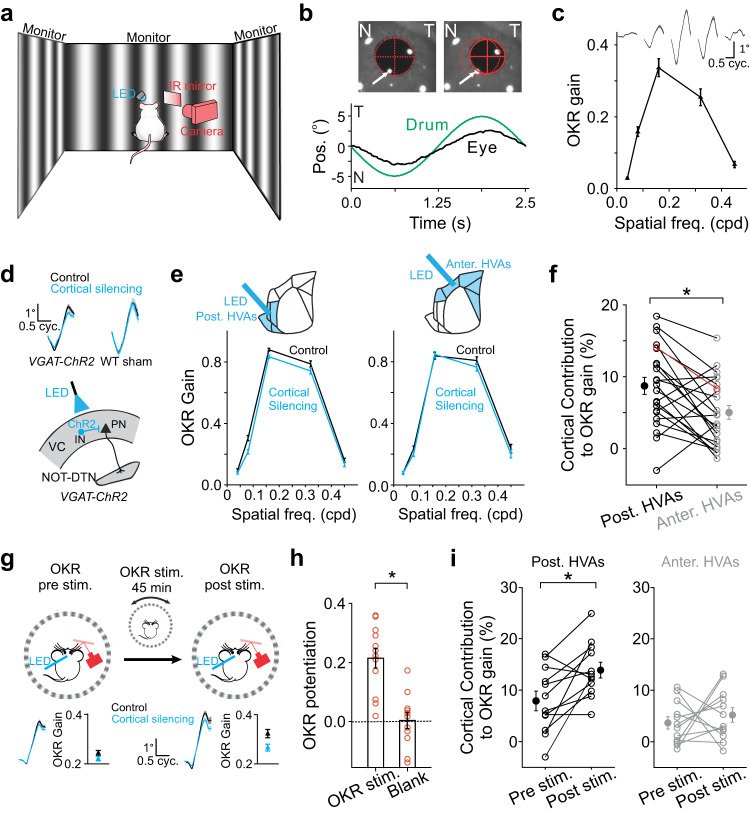
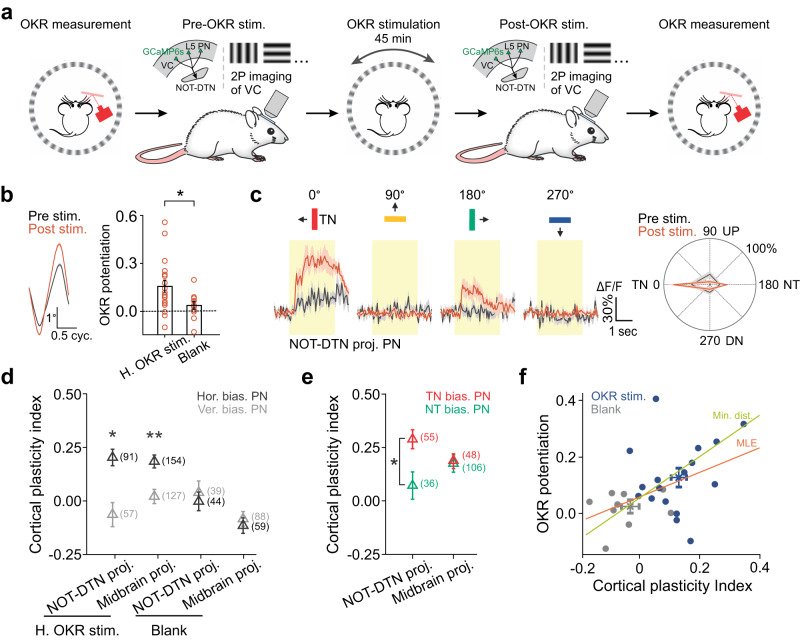
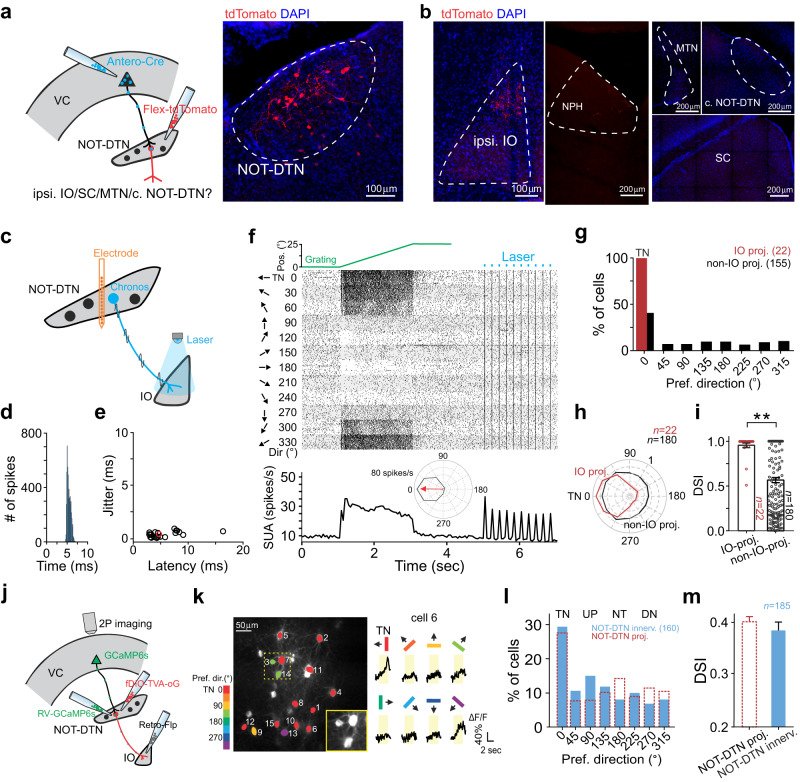
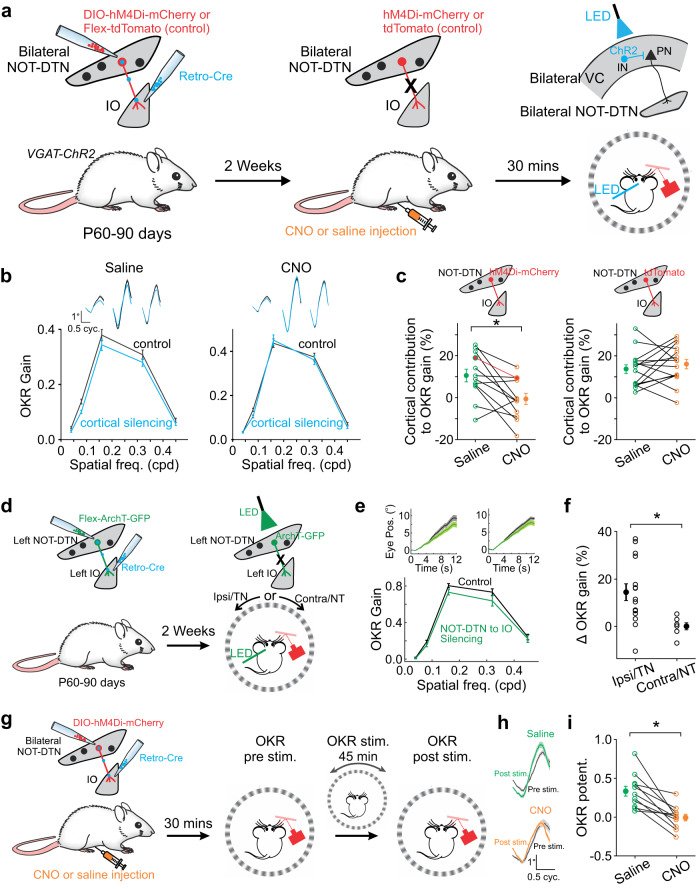
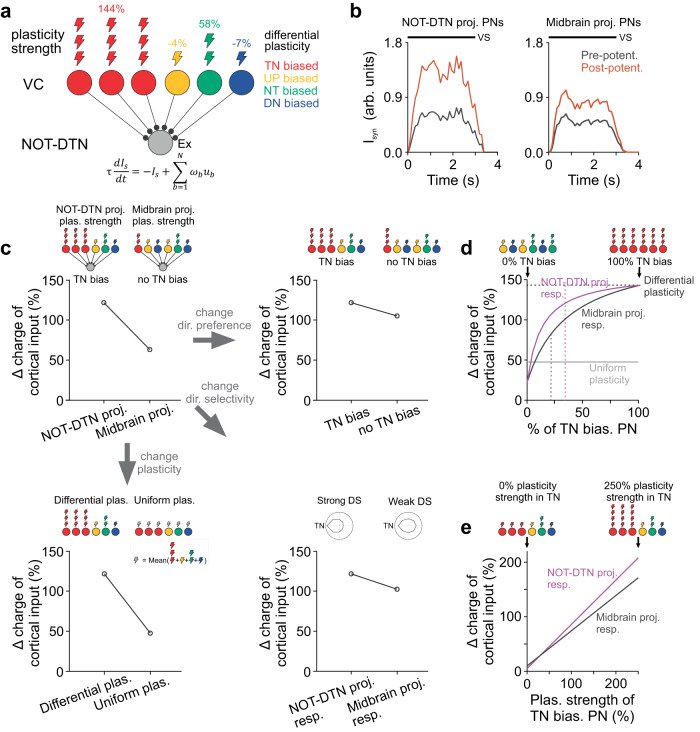
Sensory cortices modulate innate behaviors through corticofugal projections targeting phylogenetically-old brainstem nuclei. However, the principles behind the functional connectivity of these projections remain poorly understood. Here, we show that in mice visual cortical neurons projecting to the optic-tract and dorsal-terminal nuclei (NOT-DTN) possess distinct response properties and anatomical connectivity, supporting the adaption of an essential innate eye movement, the optokinetic reflex (OKR). We find that these corticofugal neurons are enriched in specific visual areas, and they prefer temporo-nasal visual motion, matching the direction bias of downstream NOT-DTN neurons. Remarkably, continuous OKR stimulation selectively enhances the activity of these temporo-nasally biased cortical neurons, which can efficiently promote OKR plasticity. Lastly, we demonstrate that silencing downstream NOT-DTN neurons, which project specifically to the inferior olive-a key structure in oculomotor plasticity, impairs the cortical modulation of OKR and OKR plasticity. Our results unveil a direction-selective cortico-brainstem pathway that adaptively modulates innate behaviors.
September 2021
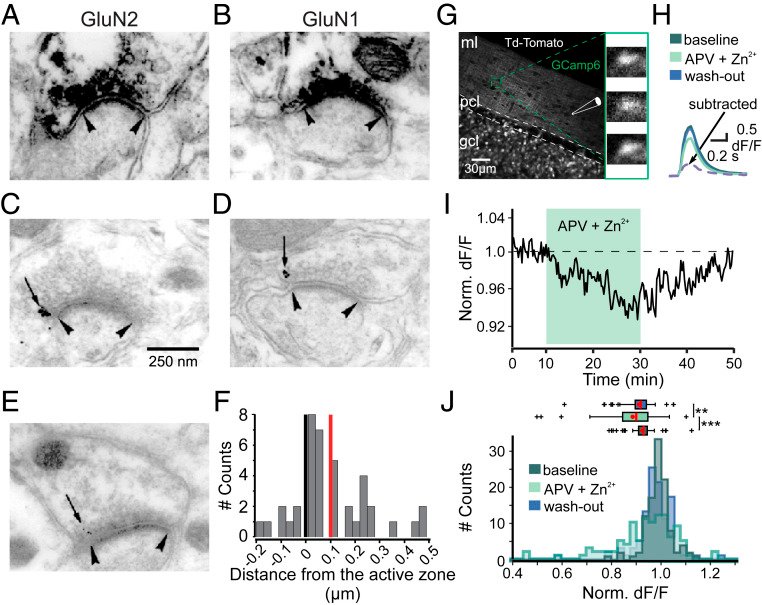
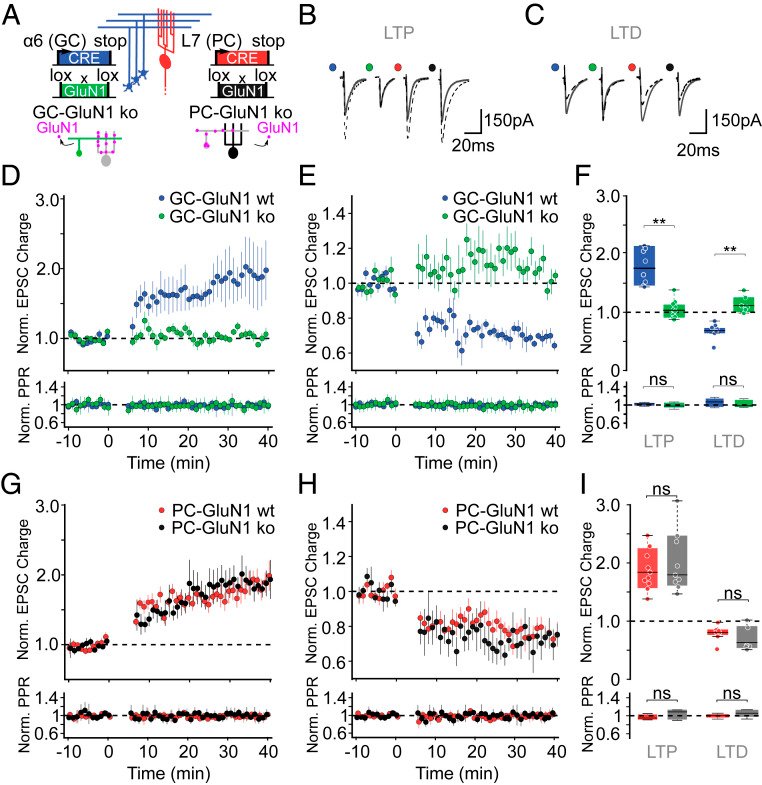
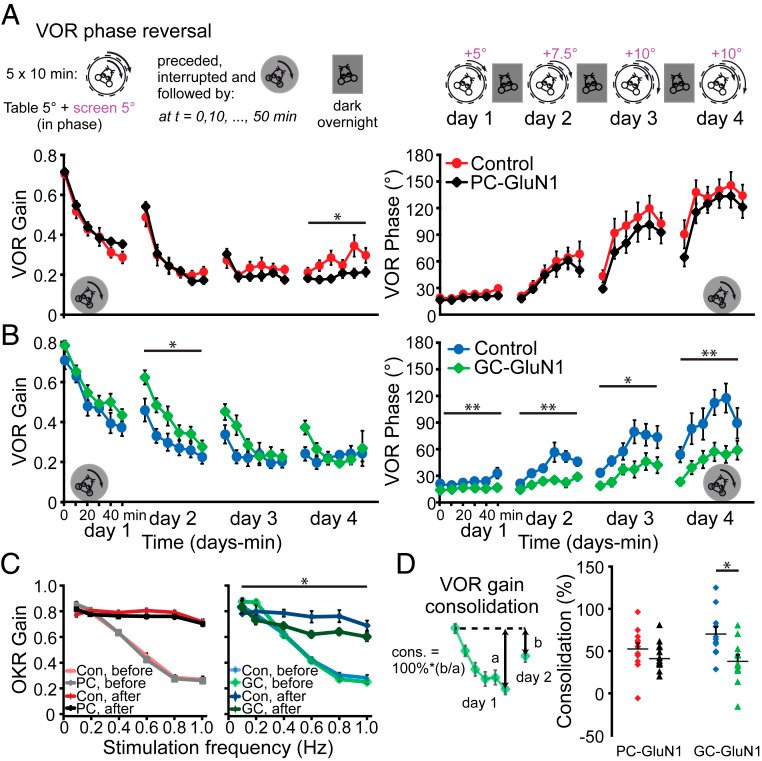
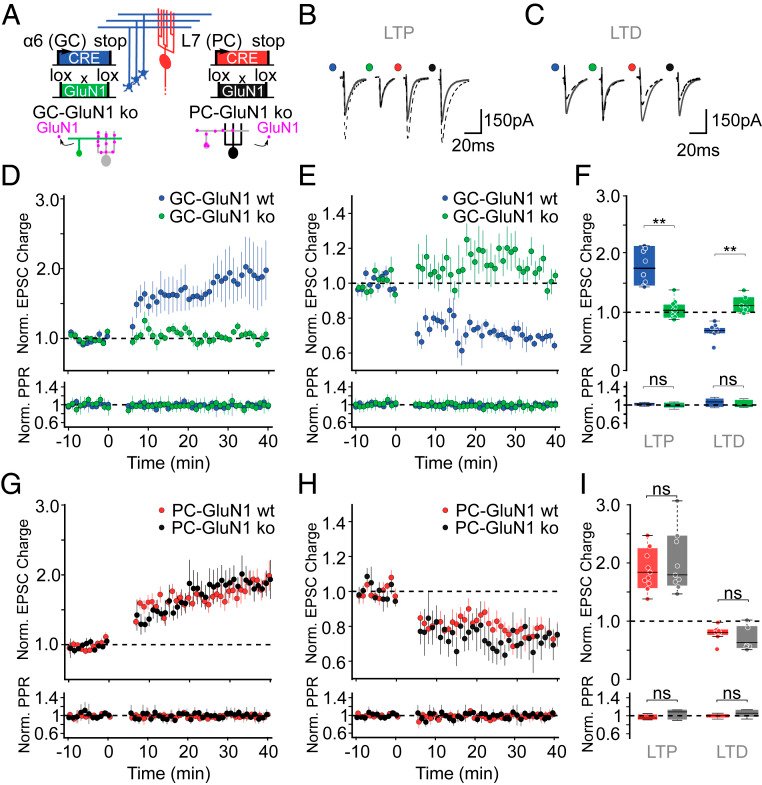
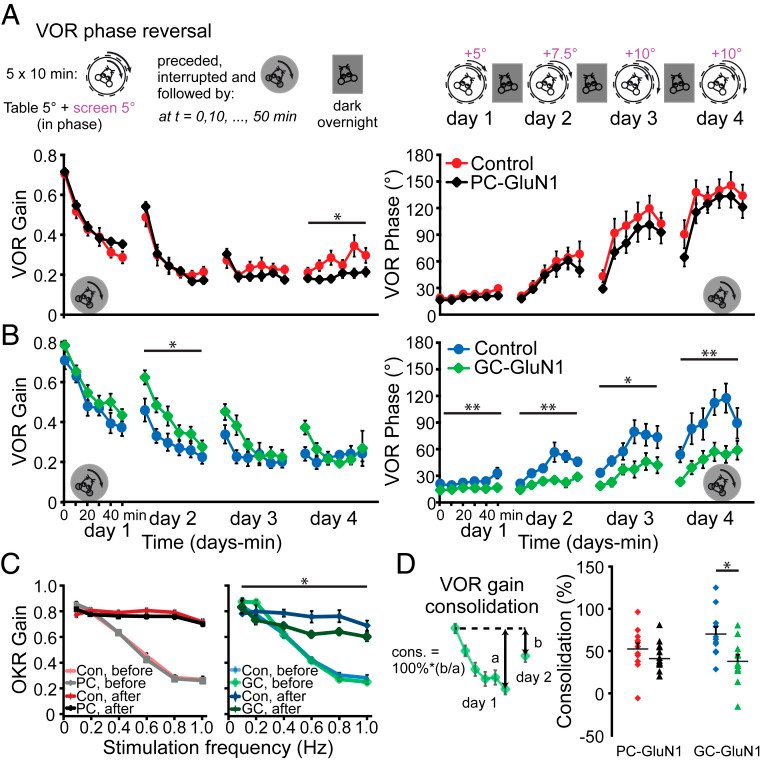
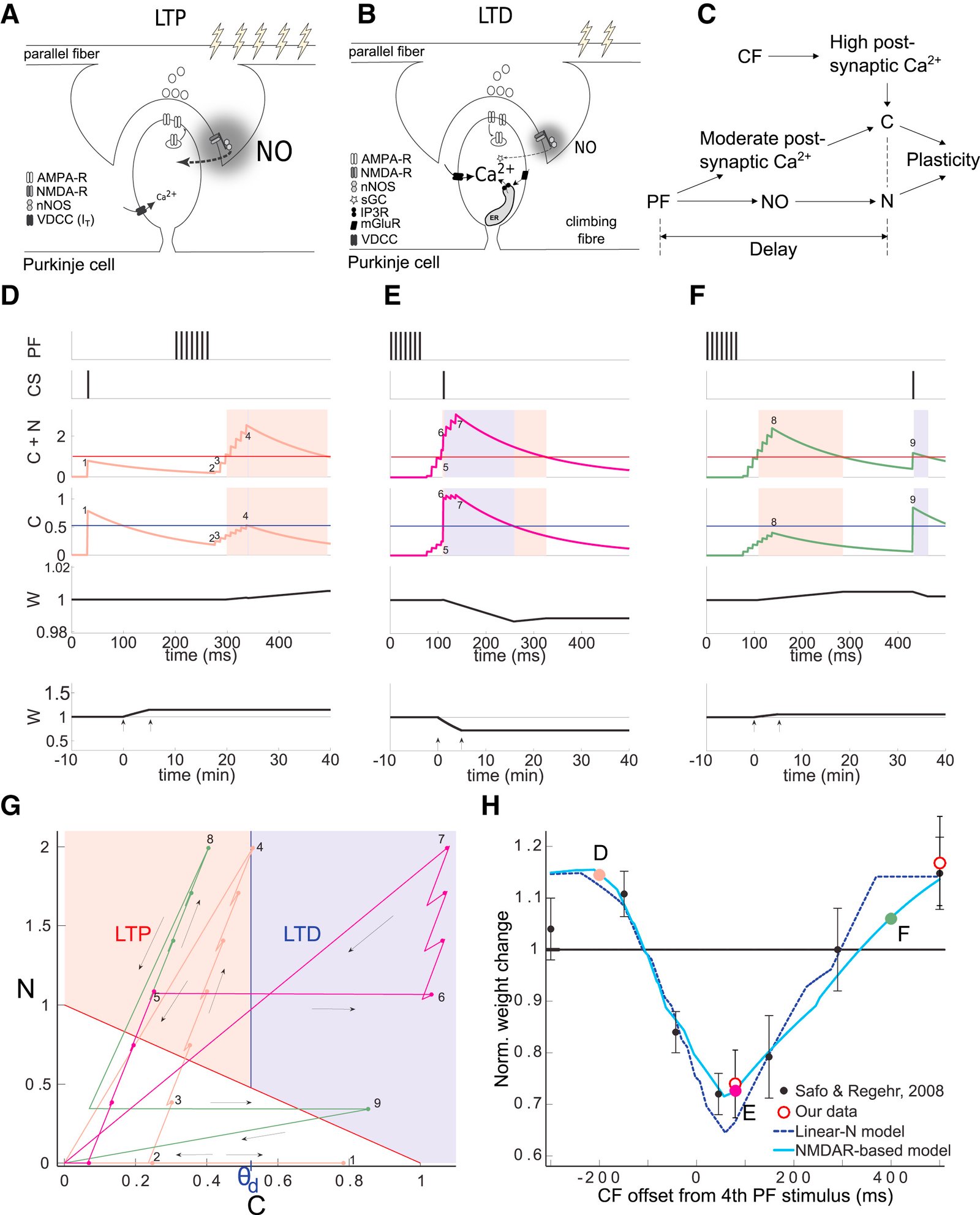
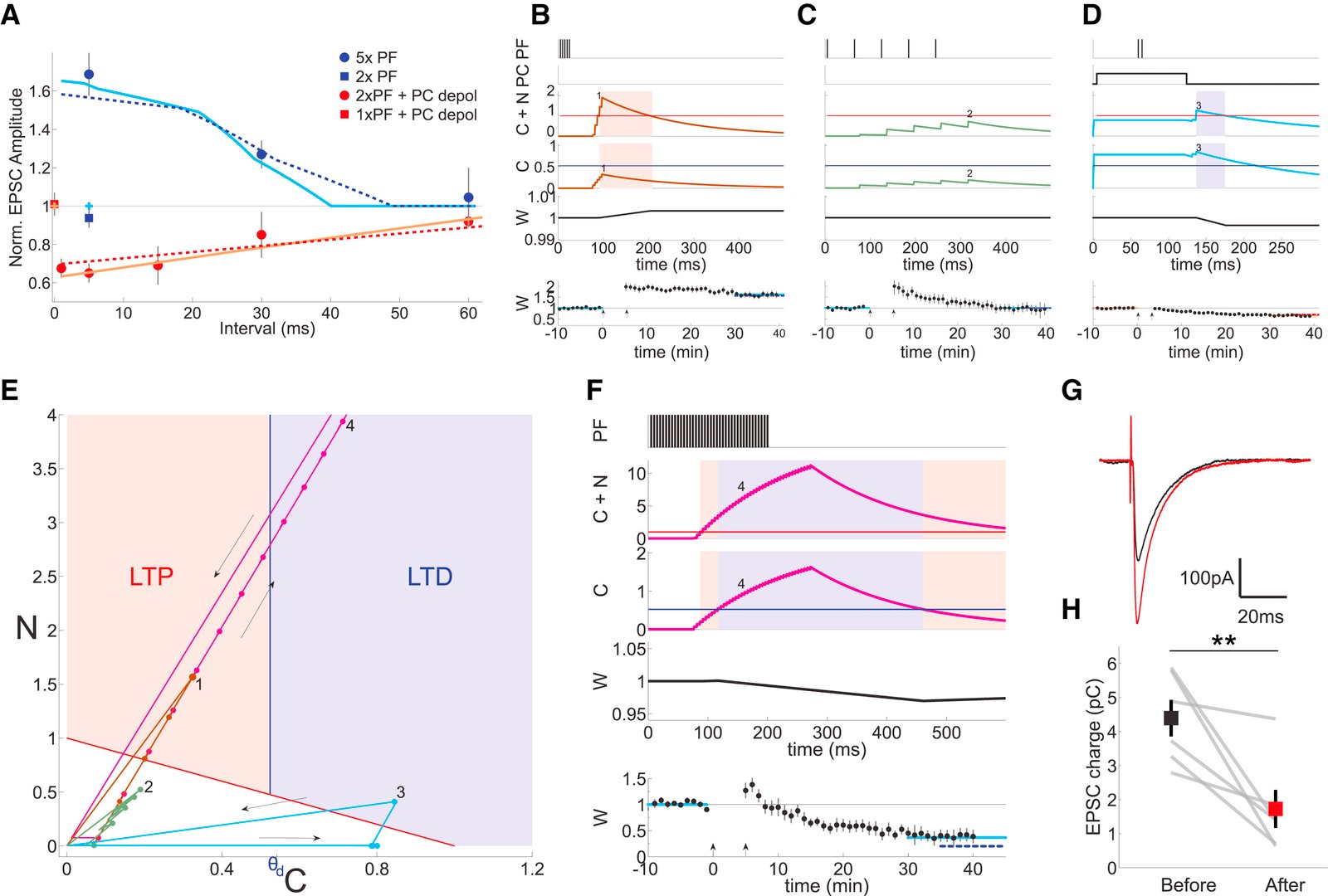
Long-term synaptic plasticity is believed to be the cellular substrate of learning and memory. Synaptic plasticity rules are defined by the specific complement of receptors at the synapse and the associated downstream signaling mechanisms. In young rodents, at the cerebellar synapse between granule cells (GC) and Purkinje cells (PC), bidirectional plasticity is shaped by the balance between transcellular nitric oxide (NO) driven by presynaptic N-methyl-D-aspartate receptor (NMDAR) activation and postsynaptic calcium dynamics. However, the role and the location of NMDAR activation in these pathways is still debated in mature animals. Here, we show in adult rodents that NMDARs are present and functional in presynaptic terminals where their activation triggers NO signaling. In addition, we find that selective genetic deletion of presynaptic, but not postsynaptic, NMDARs prevents synaptic plasticity at parallel fiber-PC (PF-PC) synapses. Consistent with this finding, the selective deletion of GC NMDARs affects adaptation of the vestibulo-ocular reflex. Thus, NMDARs presynaptic to PCs are required for bidirectional synaptic plasticity and cerebellar motor learning.
November 2020
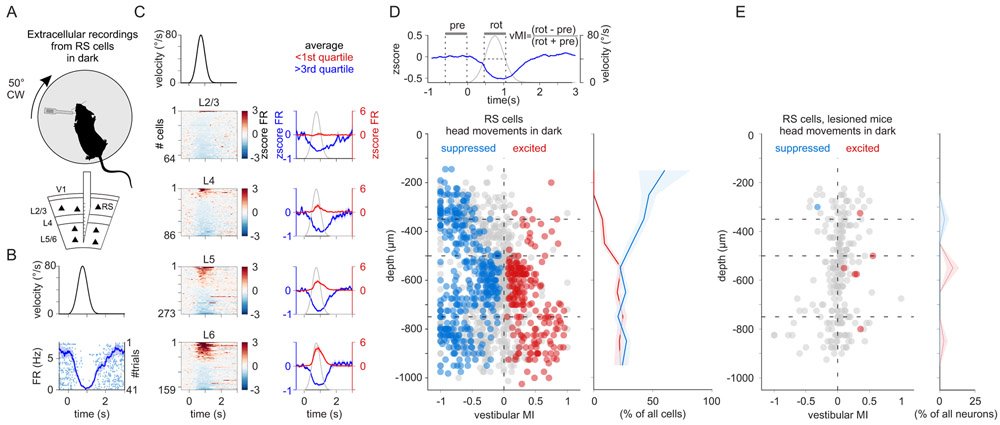
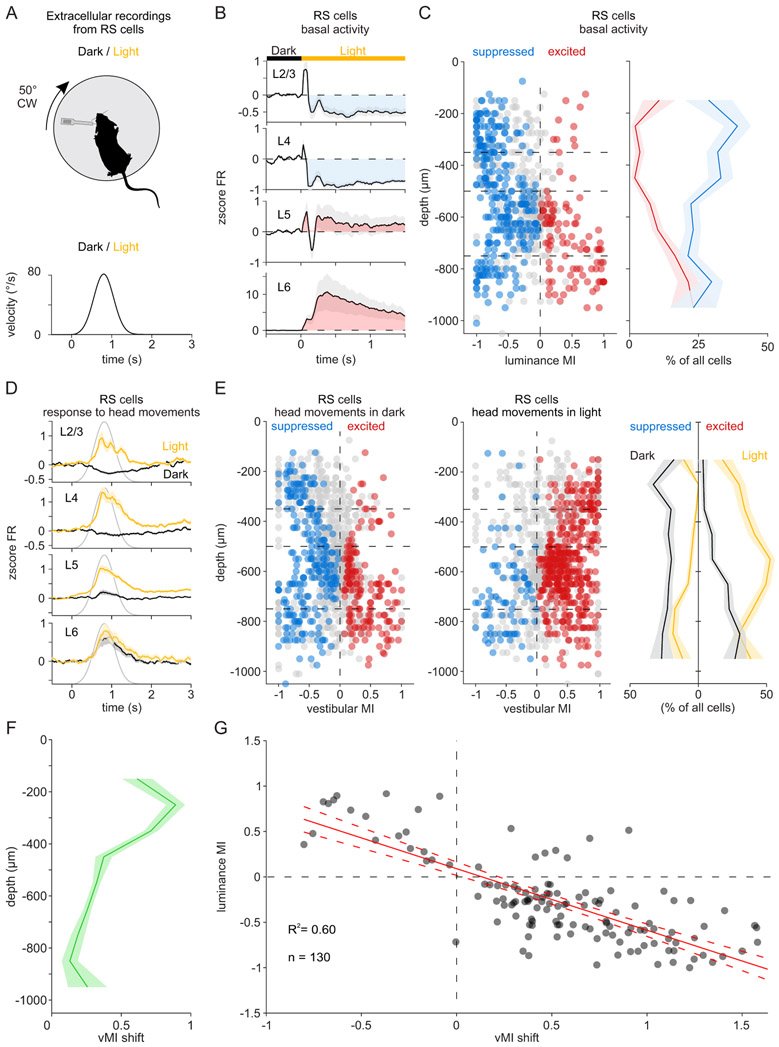
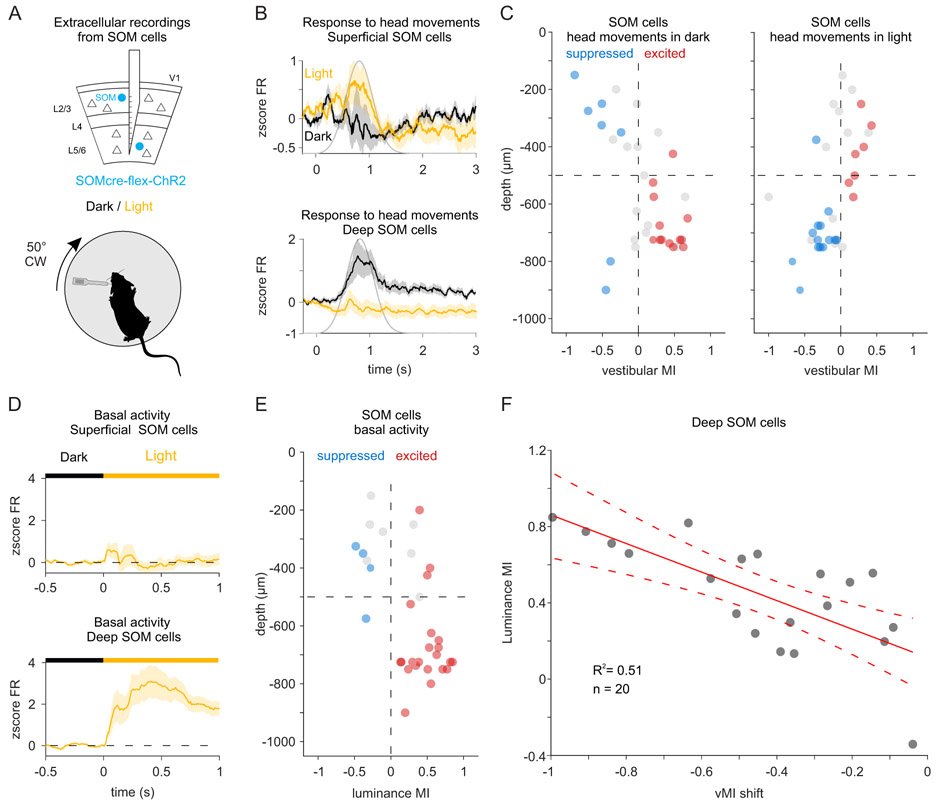
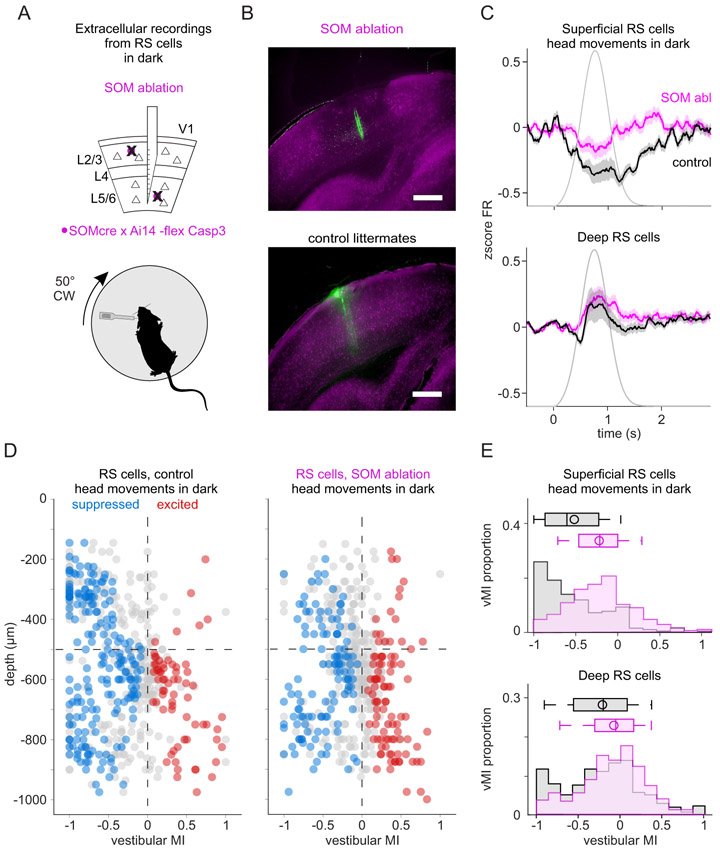
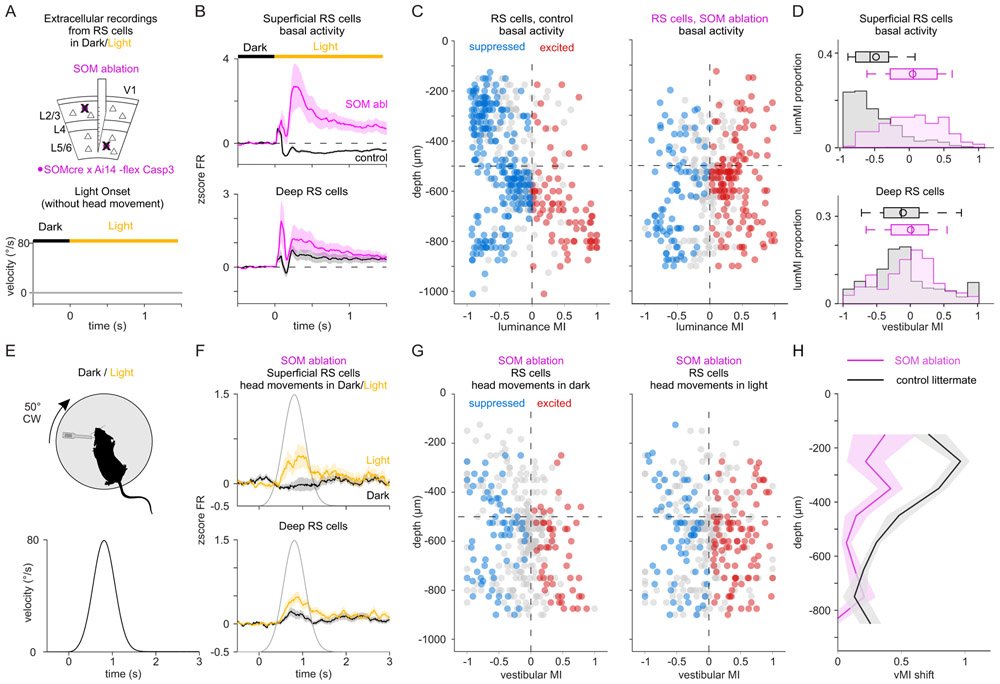
The vestibular system broadcasts head-movement-related signals to sensory areas throughout the brain, including visual cortex. These signals are crucial for the brain's ability to assess whether motion of the visual scene results from the animal's head movements. However, how head movements affect visual cortical circuits remains poorly understood. Here, we discover that ambient luminance profoundly transforms how mouse primary visual cortex (V1) processes head movements. While in darkness, head movements result in overall suppression of neuronal activity; in ambient light, the same head movements trigger excitation across all cortical layers. This light-dependent switch in how V1 processes head movements is controlled by somatostatin-expressing (SOM) inhibitory neurons, which are excited by head movements in dark, but not in light. This study thus reveals a light-dependent switch in the response of V1 to head movements and identifies a circuit in which SOM cells are key integrators of vestibular and luminance signals.
Articles
Advanced materials
July 2025
Intracellular recordings provide unique access to the submillisecond neuronal membrane potential changes, revealing dynamics that orchestrate cellular, local, and large-scale brain activity. However, technical requirements limit the scalability of intracellular recordings to large populations of neurons, especially within intact brains. To overcome this limitation, a Fishbone Intracellular Nanowire Electrode (FINE) is developed with ultra-sharp nanowire tips strategically integrated at slanted angles along an implantable shank to record 3D intracellular potentials from ensembles of neurons in intact brain. A novel fabrication process is developed to integrate reverse-angled platinum silicide (PtSi) nanowires to preserve the structural integrity of FINE during insertion. As-implanted or sub-micron retraced FINE spreads the PtSi nanowires away from the shank to establish intimate nanowire-neuron interfaces that yield quasi-intracellular potentials. Comparative analyses of nanowire recordings versus adjacent planar recordings on the same shank validate their distinctive quasi-intracellular recording characteristics. The scalability of FINE is demonstrated to a 3D 24-shank array with 594 nanowires and 430 planar contacts and successfully identified quasi-intracellular potentials across 127 distinct nanowires in the intact brain. FINE's 3D quasi-intracellular recording holds the potential to unlock detailed investigations of the intricate ionic potential fluctuations and patterns of transmembrane potentials that drive behavior and cognition.
October 2024
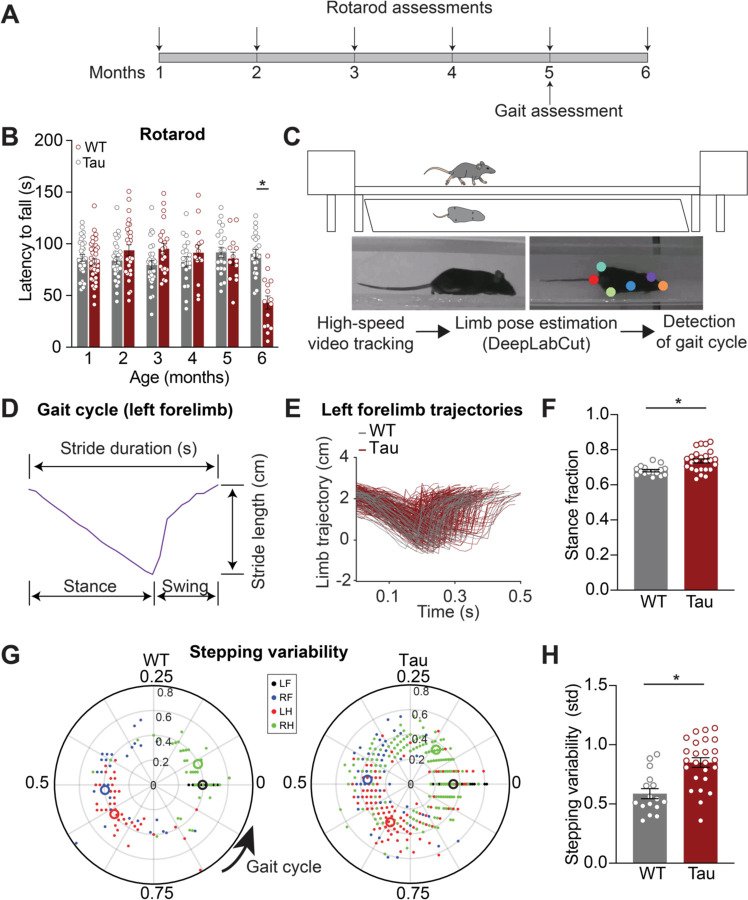
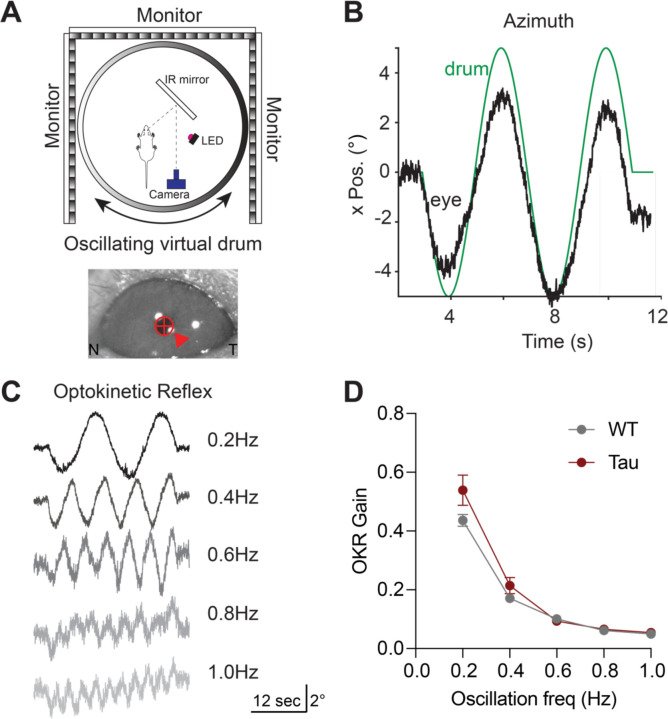
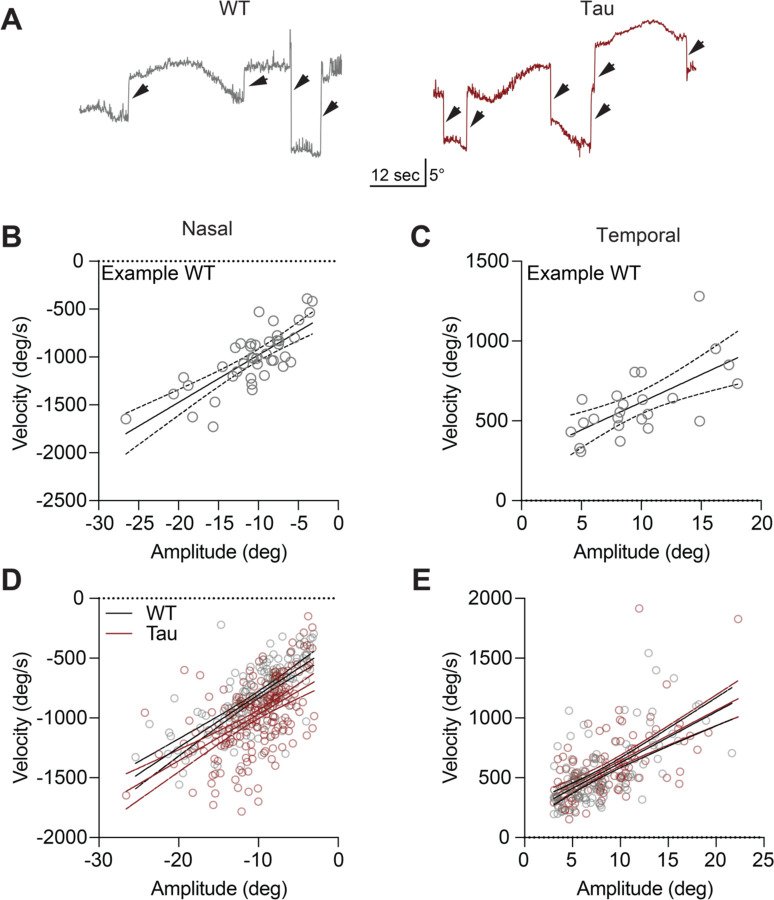
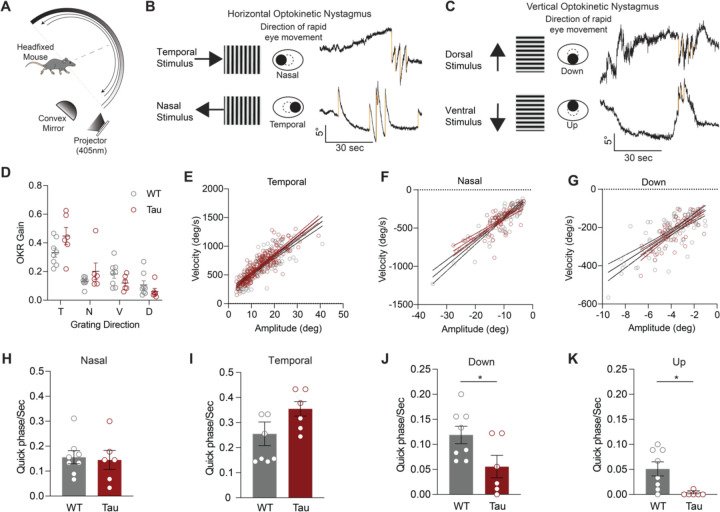
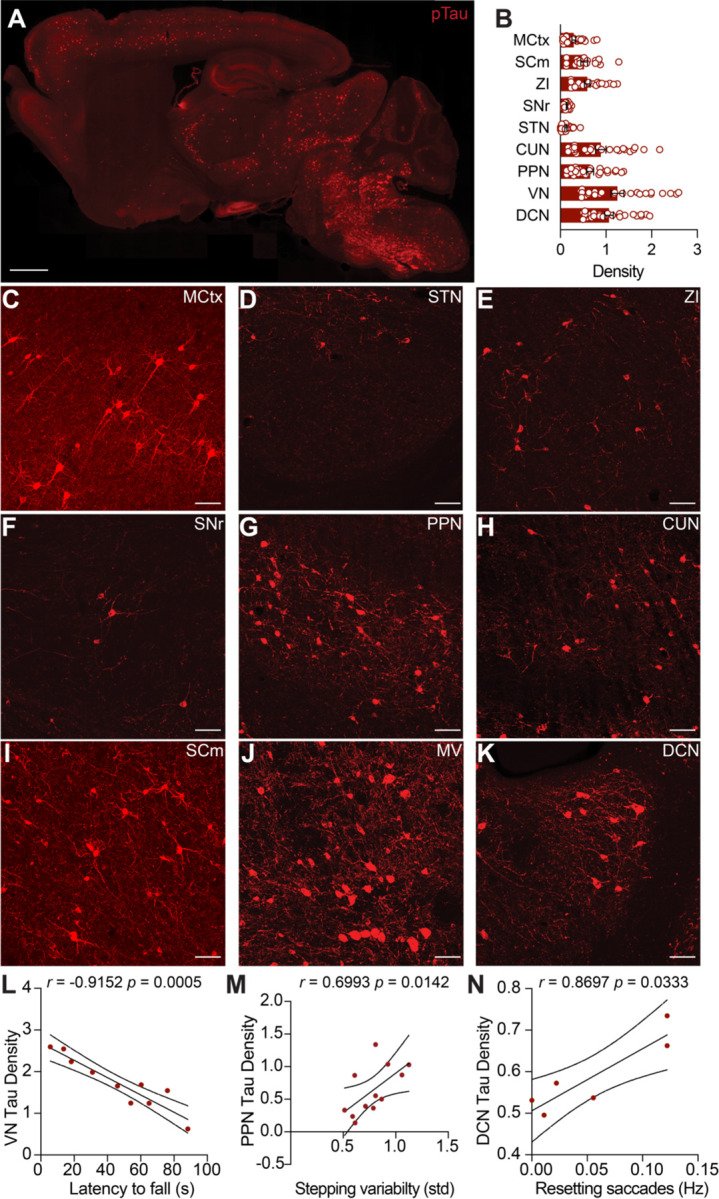
Progressive supranuclear palsy (PSP) is a neurodegenerative disorder with an estimated prevalence of 5–7 people in 100,000. Clinically characterized by impairments in gait, balance, and eye movements, as well as aggregated Tau pathology, PSP leads to death in approximately 5–8 years. No disease-modifying treatments are currently available. The contribution of Tau pathology to the symptoms of patients with PSP is poorly understood, in part due to lack of a rodent model that recapitulates characteristic aspects of PSP. Here, we assessed the hTau.P301S mouse for key clinical features of PSP, finding progressive impairments in balance and gait coordination. Additionally, we found impairments in fast vertical eye movements, one of the most distinctive features of PSP. Across animals, we found that Tau pathology in motor control regions correlated with motor deficits. These findings highlight the utility of the hP301S mouse in modeling key aspects of PSP.
February 2022
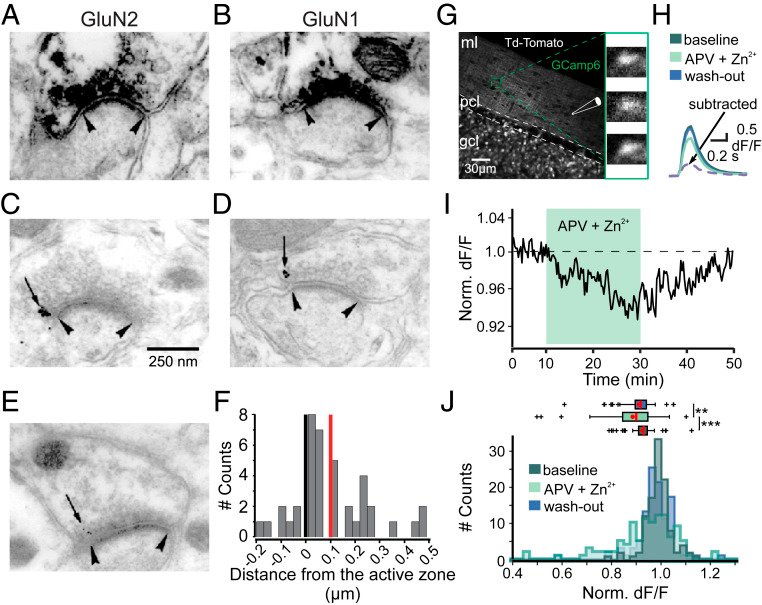
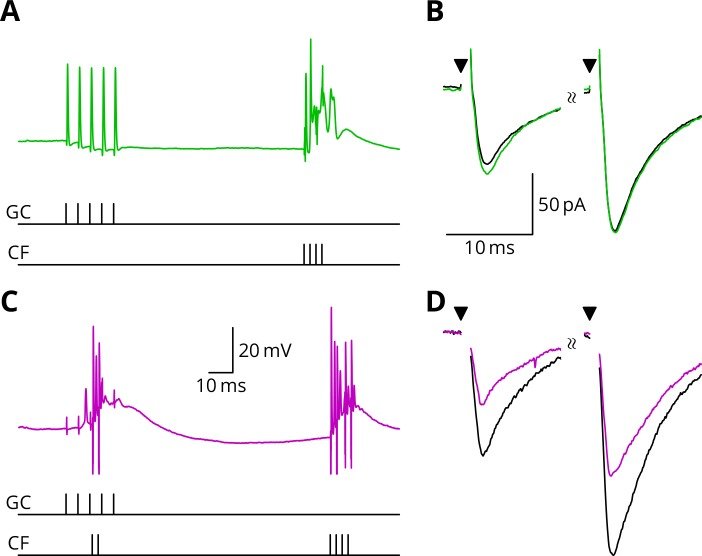
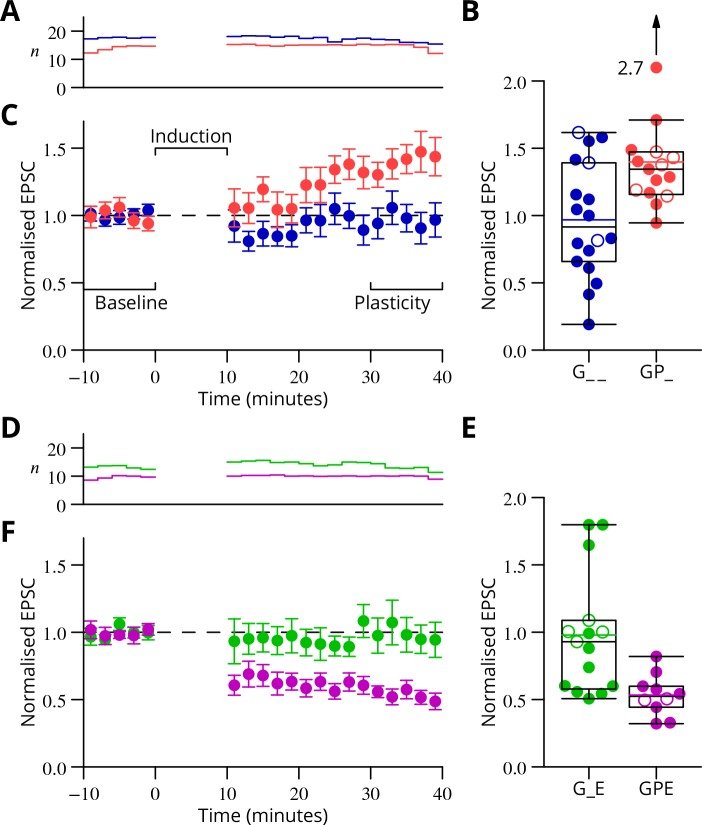
In our study, we presented functional and immunohistochemical evidence for the presence of presynaptic N-methyl-D-aspartate receptors (pre-NMDARs) at parallel fiber–Purkinje cell (PF-PC) synapses in the adult cerebellum. As in young rodents, pre-NMDARs are required to induce PF-PC synaptic plasticity. Using cell-specific deletion of NMDARs in granule cells (GCs) or PCs, we demonstrated that only GC NMDARs are robustly involved in PF-PC synaptic plasticity and vestibuloocular reflex (VOR) adaptation.
Our data contradict those by Piochon et al., who proposed that PC NMDARs initiate PF-PC long-term depression (LTD). The question remains to what extent PC NMDARs at climbing fiber (CF) synapses may affect PF synapses. The amplitude of CF-PC NMDA currents is orders of magnitude smaller than the AMPA currents that cause a depolarized plateau. It is thus unlikely that CF-PC NMDARs can directly affect electrogenesis during complex spikes. However, blockage of CF-PC NMDARs could still affect PF-PC LTD through long-term effects on PC excitability and CF dendritic spikes or CF-PC LTD.
Piochon et al. partly based their conclusions on blocking PC NMDARs with intracellular MK801 and performing direct PF stimulation in sagittal slices at high calcium concentrations. Since the MK801 concentration used in the intracellular medium is 1,000 times higher than the concentration required in an extracellular medium, MK801 may have leaked out of the cell (or the pipette prior to patching), blocking pre-NMDARs on PFs. Moreover, their stimulation configuration may have bypassed involvement of pre-NMDARs in LTP induction and changed the plasticity rule by perturbing presynaptic calcium dynamics. We, instead, used cell type–specific genetic deletions of NMDARs and transverse slices that allowed PF stimulation far away from the recording site, precluding the caveats described above.
Consistent with the concept that we proposed, Piochon et al. suggest that, when using physiological concentrations of divalent cations, similar to the one in our study, LTD requires clusters of complex spikes. However, not only the stimulus pattern of CF activity but also the presynaptic stimulation conditions should optimally match the in vivo situation. They used direct PF stimulation in sagittal slices that activates a bundle of axons, which is unlikely to occur in vivo and could affect PF-PC LTD induction.
With respect to the behavioral experiments, our previous work on mutant mouse lines, in which LTD is ablated, has indeed indicated that PF-PC LTD is not essential for VOR adaptation. Instead, PF-PC LTP and concomitant simple spike increases in firing rate appear to provide an essential contribution to VOR adaptation. Given that not only PF-PC LTD but also LTP is affected in the pre-NMDAR GC-GluN1 mice, we selected a behavioral paradigm that is linked to a lack of LTP. We, indeed, also found a phenotype in mice with PC-specific deletion of NMDARs, albeit subtle. As LTD was not impaired in these mice, this does not contradict our interpretation. It could reflect a role for PC NMDARs, such as in plasticity of CFs, which we did not study here.
Taken together, we appreciate the feedback of Piochon et al. and share their drive to gain more insight through further studies. However, based on our findings and the current literature, we do not see sufficient reason to change our original conclusions.
September 2021
Long-term synaptic plasticity is believed to be the cellular substrate of learning and memory. Synaptic plasticity rules are defined by the specific complement of receptors at the synapse and the associated downstream signaling mechanisms. In young rodents, at the cerebellar synapse between granule cells (GC) and Purkinje cells (PC), bidirectional plasticity is shaped by the balance between transcellular nitric oxide (NO) driven by presynaptic N-methyl-D-aspartate receptor (NMDAR) activation and postsynaptic calcium dynamics. However, the role and the location of NMDAR activation in these pathways is still debated in mature animals. Here, we show in adult rodents that NMDARs are present and functional in presynaptic terminals where their activation triggers NO signaling. In addition, we find that selective genetic deletion of presynaptic, but not postsynaptic, NMDARs prevents synaptic plasticity at parallel fiber-PC (PF-PC) synapses. Consistent with this finding, the selective deletion of GC NMDARs affects adaptation of the vestibulo-ocular reflex. Thus, NMDARs presynaptic to PCs are required for bidirectional synaptic plasticity and cerebellar motor learning.
Nature Neuroscience
May 2020
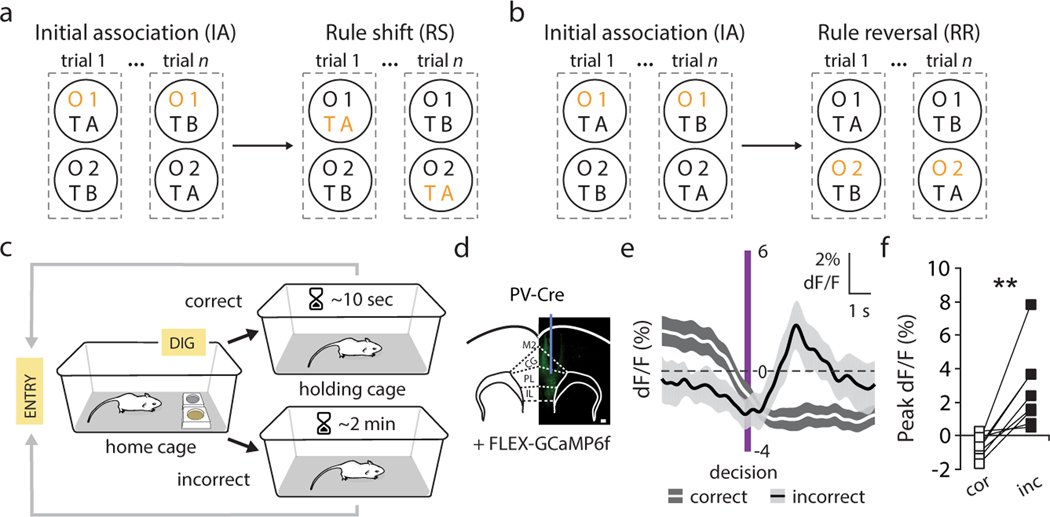
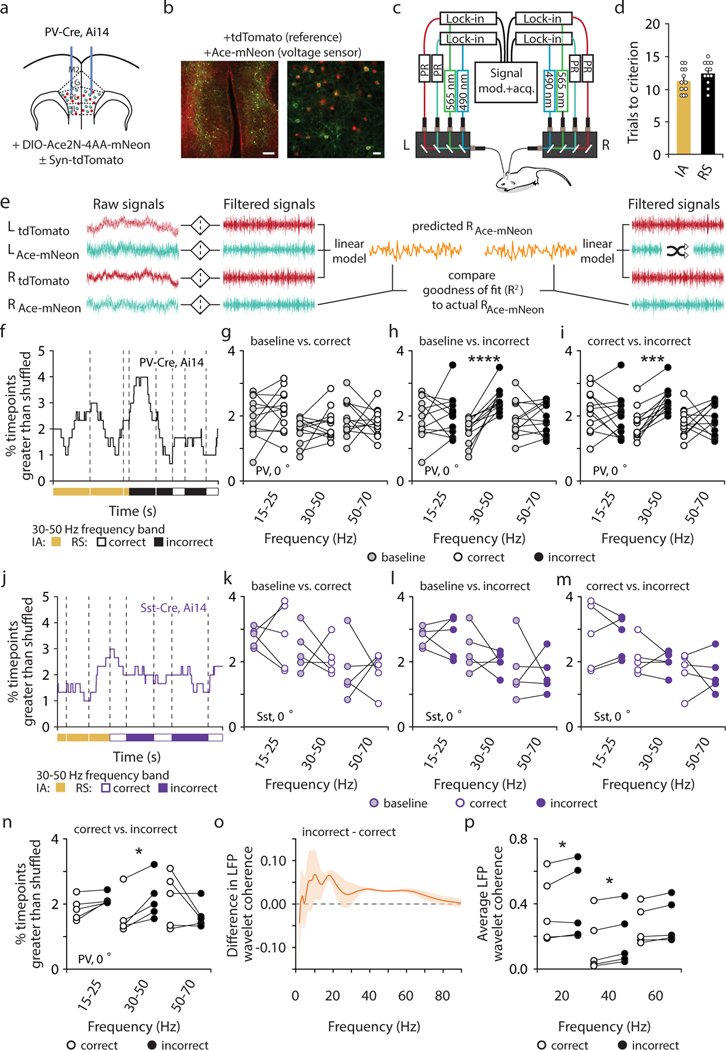
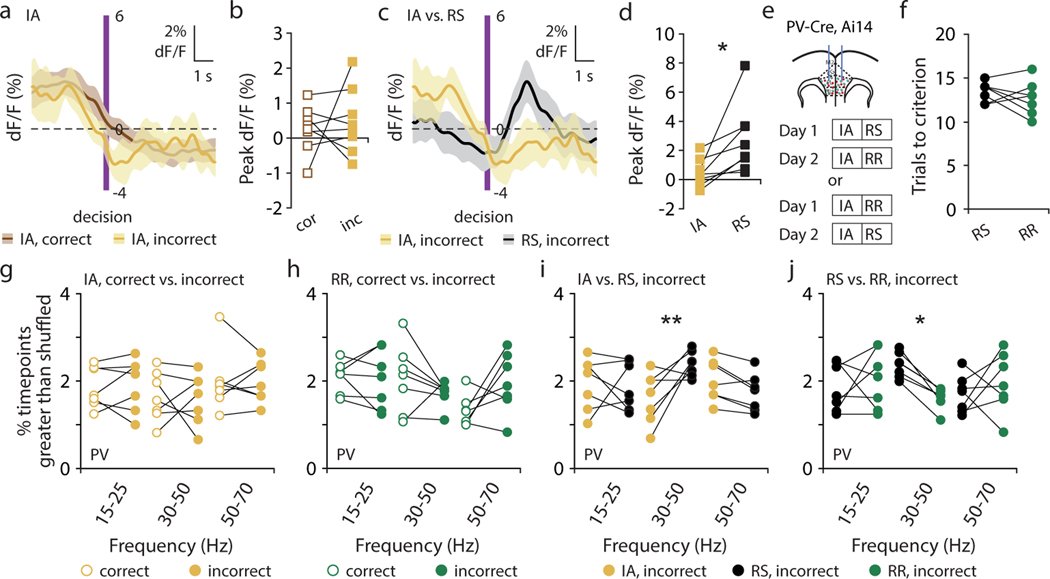
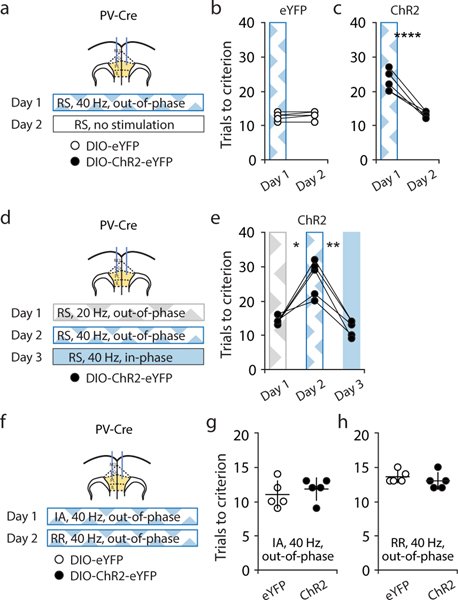
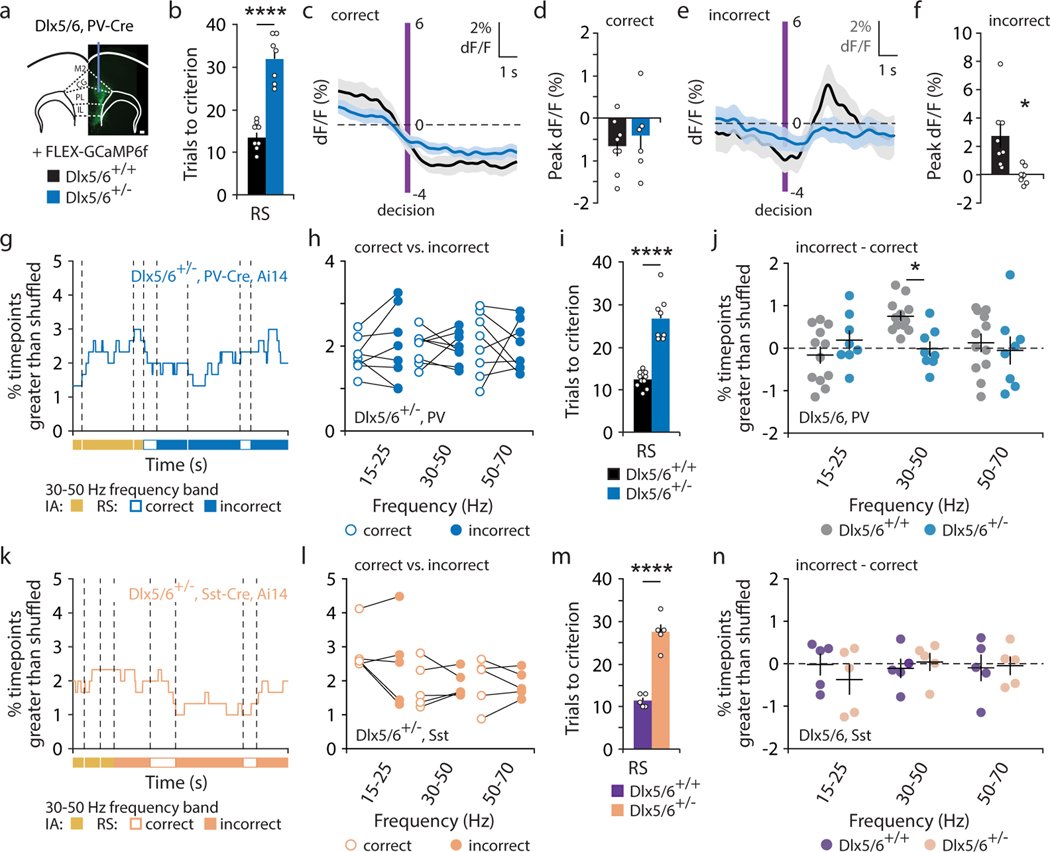
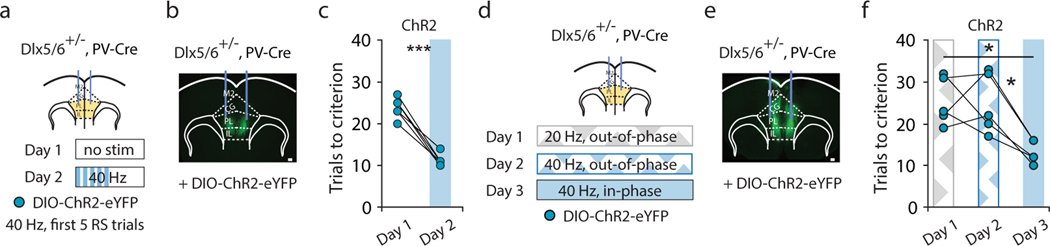
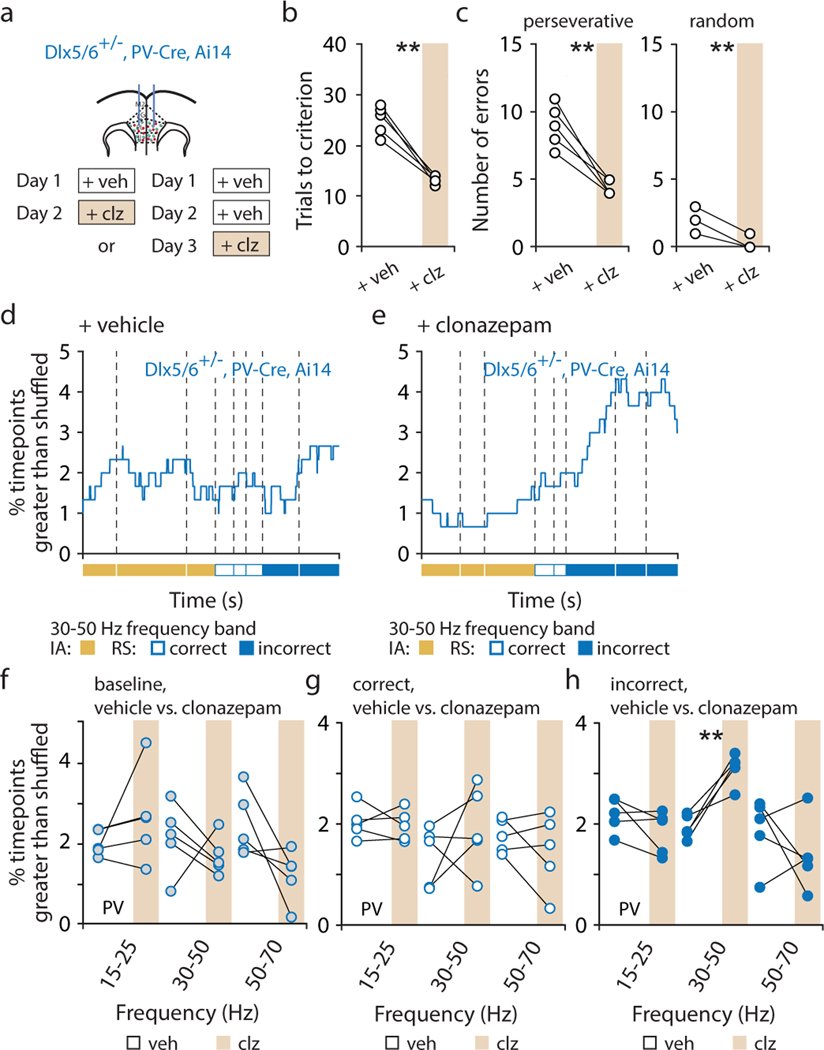
Organisms must learn new strategies to adapt to changing environments. Activity in different neurons often exhibits synchronization that can dynamically enhance their communication and might create flexible brain states that facilitate changes in behavior. We studied the role of gamma-frequency (~40 Hz) synchrony between prefrontal parvalbumin (PV) interneurons in mice learning multiple new cue–reward associations. Voltage indicators revealed cell-type-specific increases of cross-hemispheric gamma synchrony between PV interneurons when mice received feedback that previously learned associations were no longer valid. Disrupting this synchronization by delivering out-of-phase optogenetic stimulation caused mice to perseverate on outdated associations, an effect not reproduced by in-phase stimulation or out-of-phase stimulation at other frequencies. Gamma synchrony was specifically required when new associations used familiar cues that were previously irrelevant to behavioral outcomes, not when associations involved new cues or for reversing previously learned associations. Thus, gamma synchrony is indispensable for reappraising the behavioral salience of external cues.
November 2018
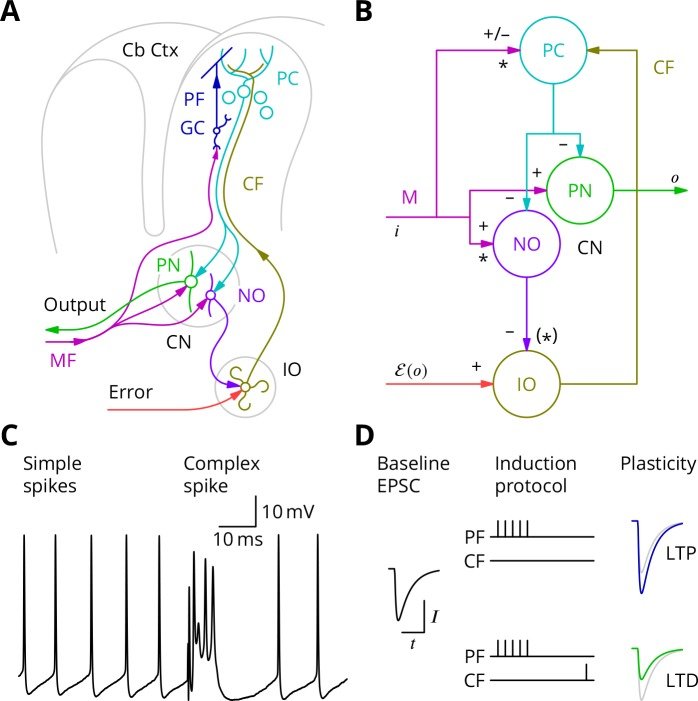
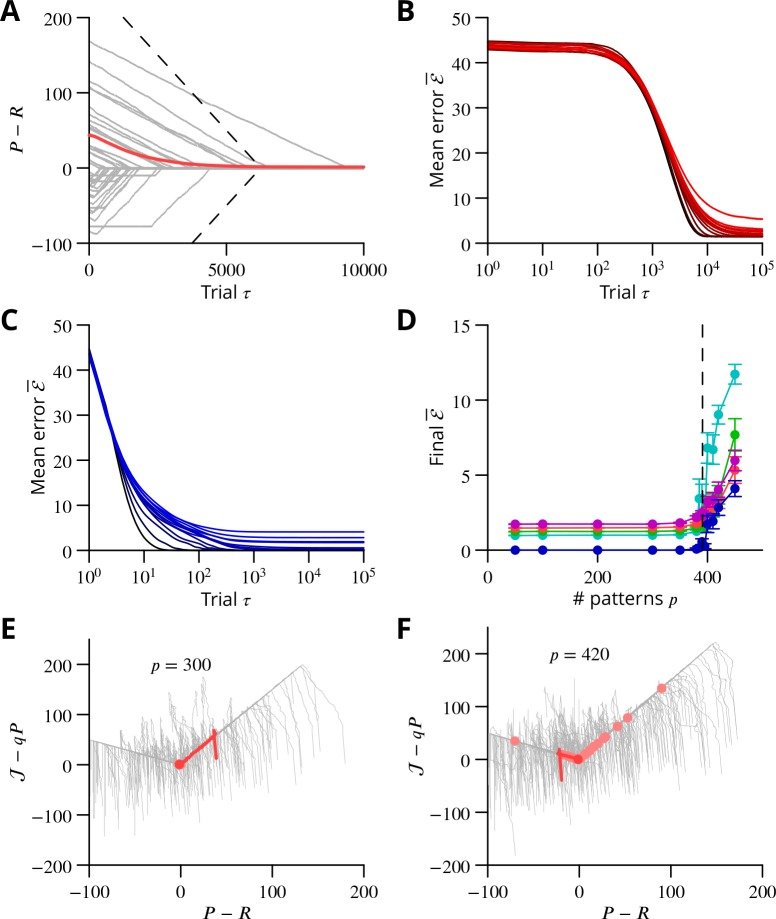
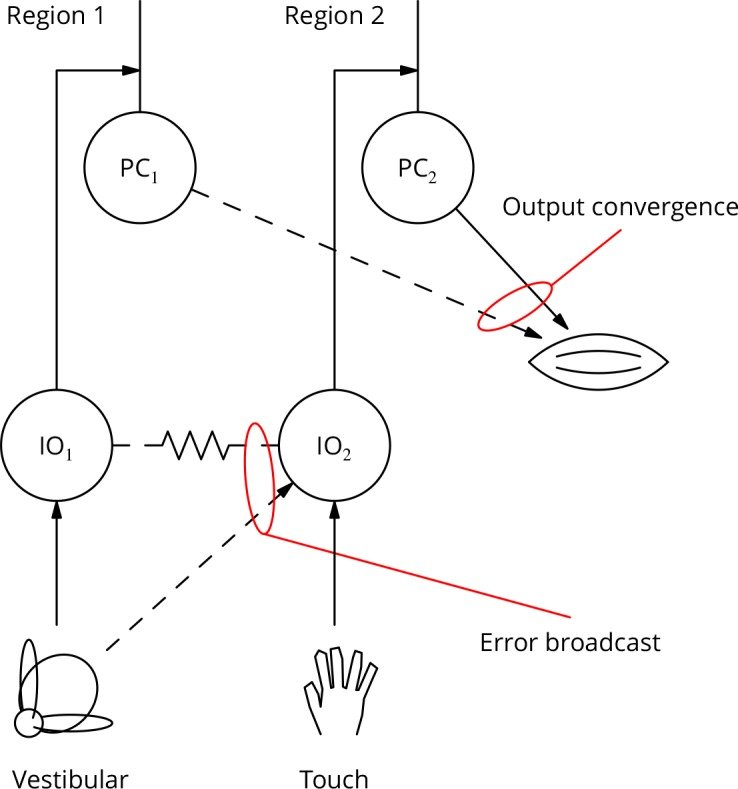
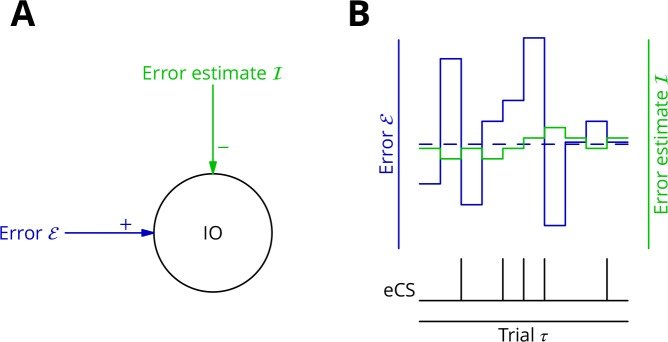
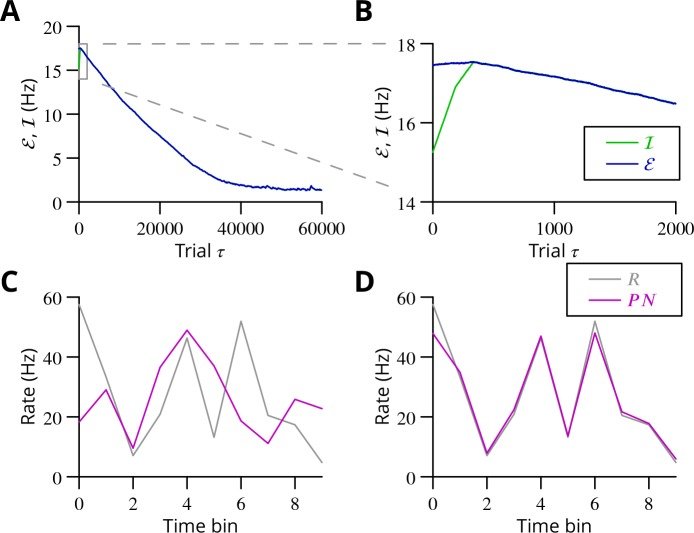
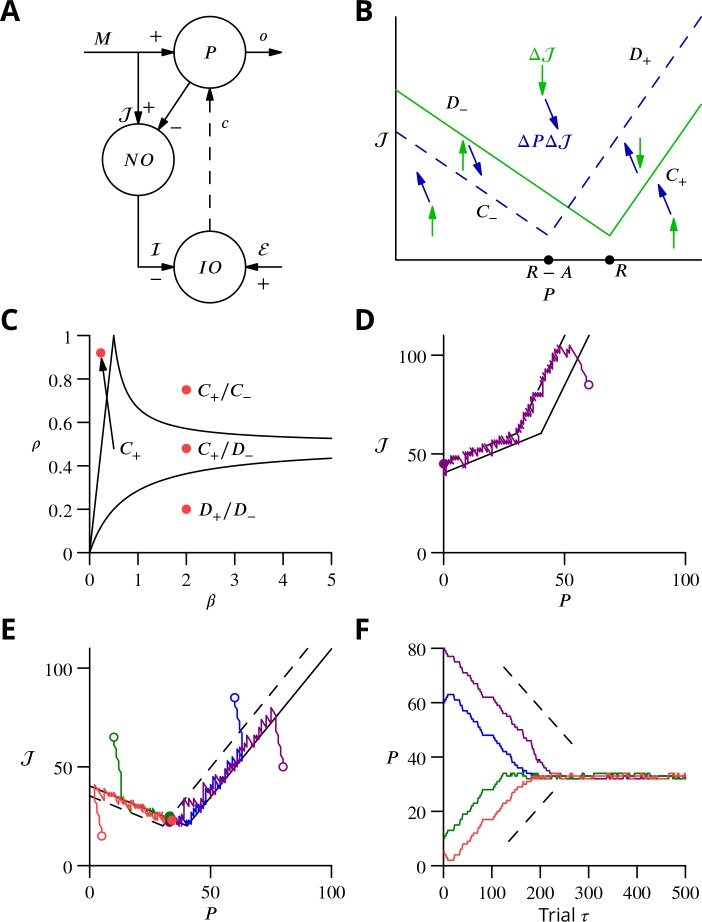
The cerebellum aids the learning of fast, coordinated movements. According to current consensus, erroneously active parallel fibre synapses are depressed by complex spikes signalling movement errors. However, this theory cannot solve the credit assignment problem of processing a global movement evaluation into multiple cell-specific error signals. We identify a possible implementation of an algorithm solving this problem, whereby spontaneous complex spikes perturb ongoing movements, create eligibility traces and signal error changes guiding plasticity. Error changes are extracted by adaptively cancelling the average error. This framework, stochastic gradient descent with estimated global errors (SGDEGE), predicts synaptic plasticity rules that apparently contradict the current consensus but were supported by plasticity experiments in slices from mice under conditions designed to be physiological, highlighting the sensitivity of plasticity studies to experimental conditions. We analyse the algorithm’s convergence and capacity. Finally, we suggest SGDEGE may also operate in the basal ganglia.
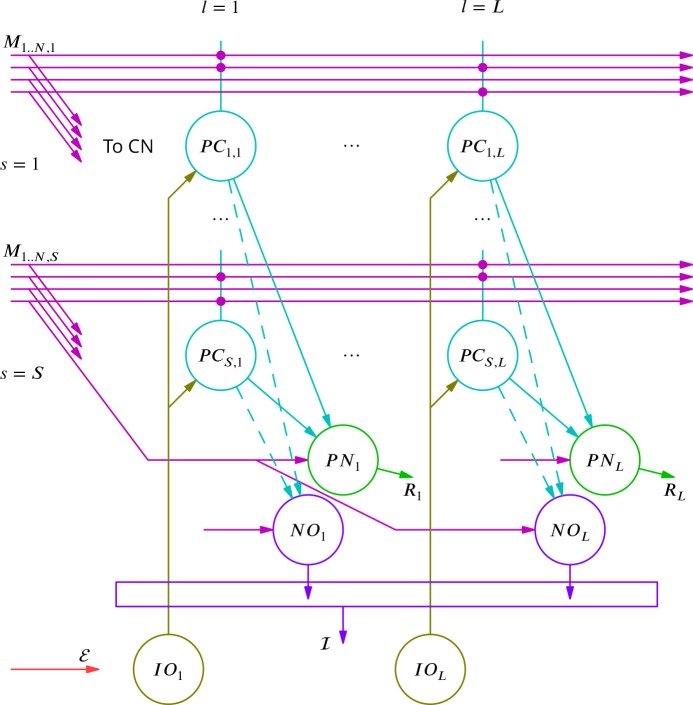
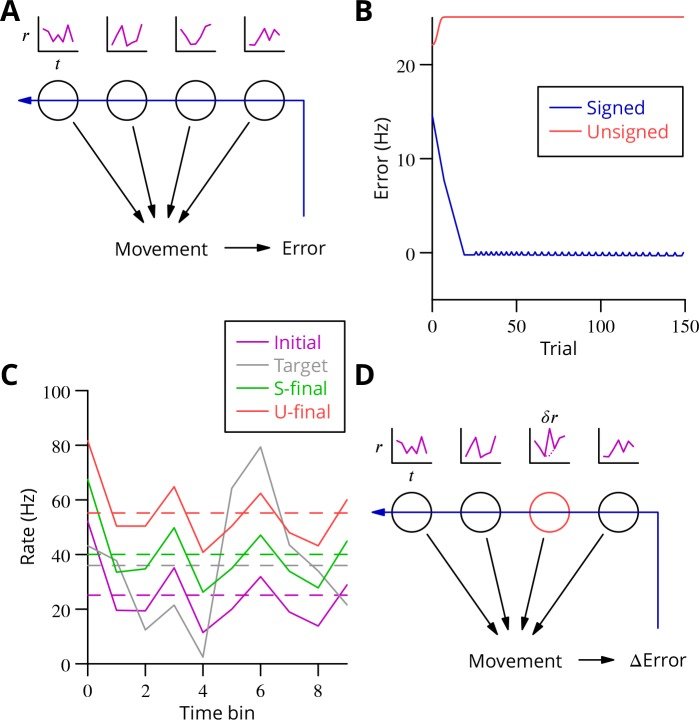
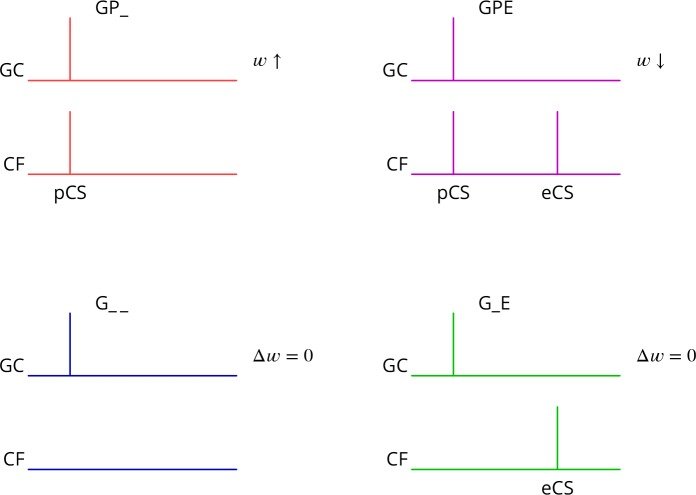
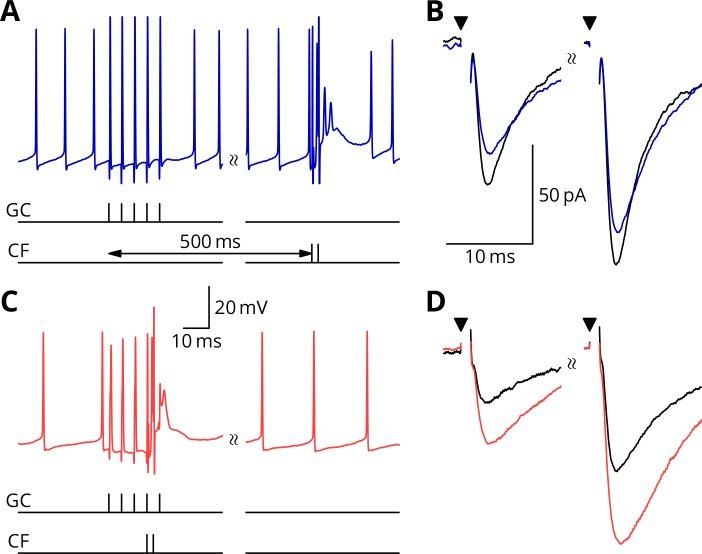
September 2016
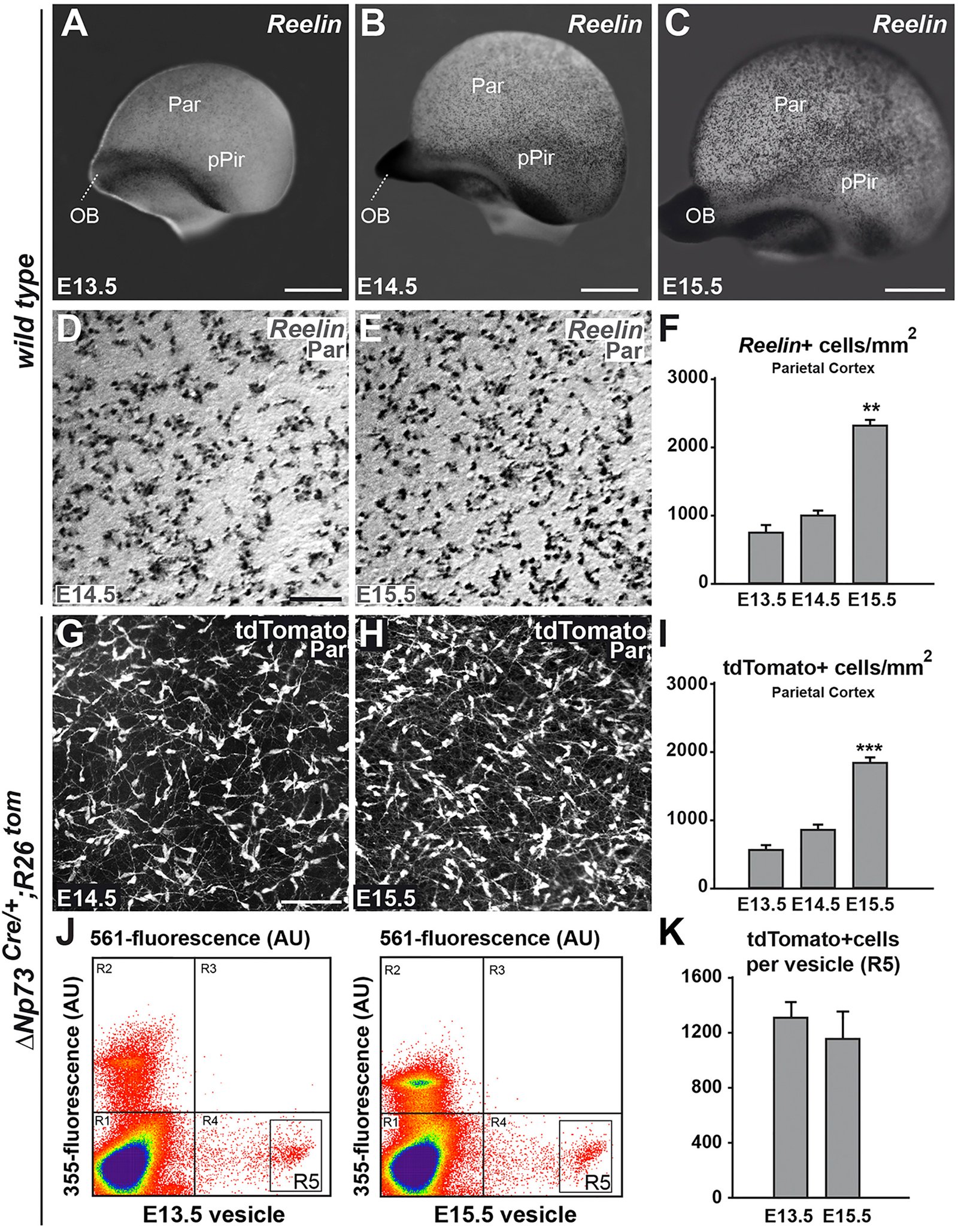
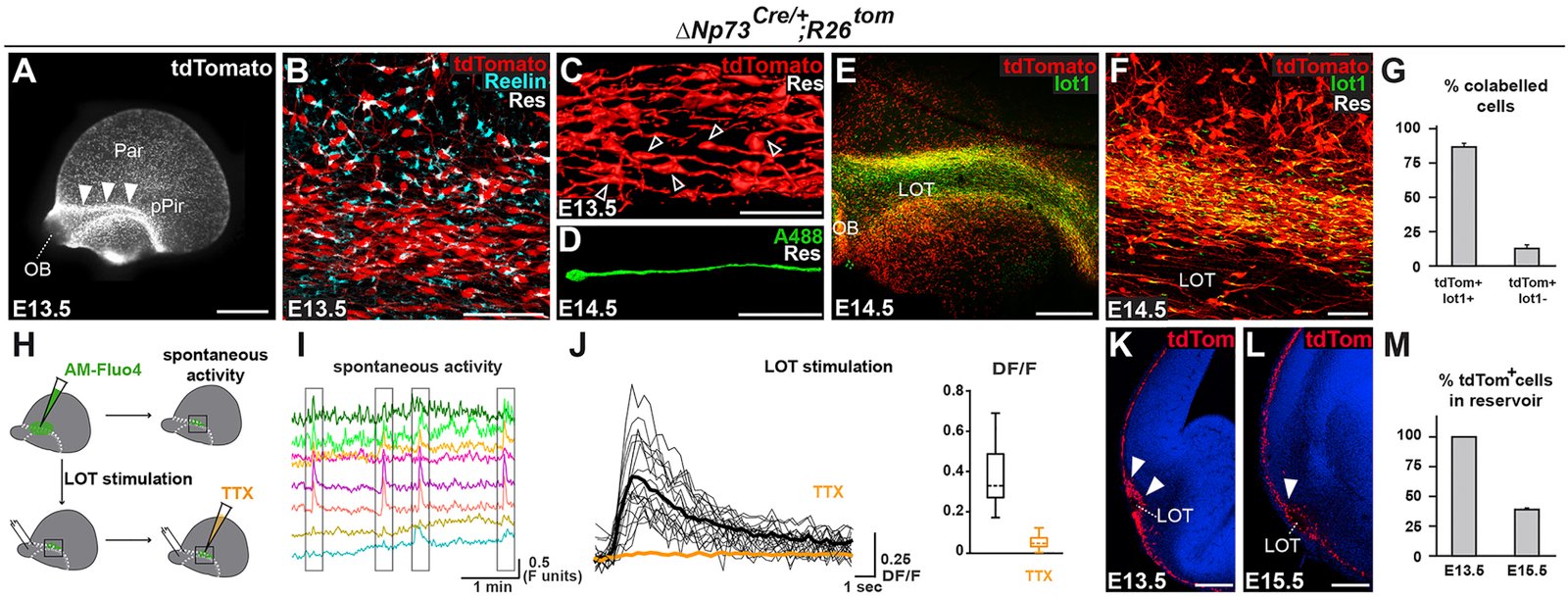
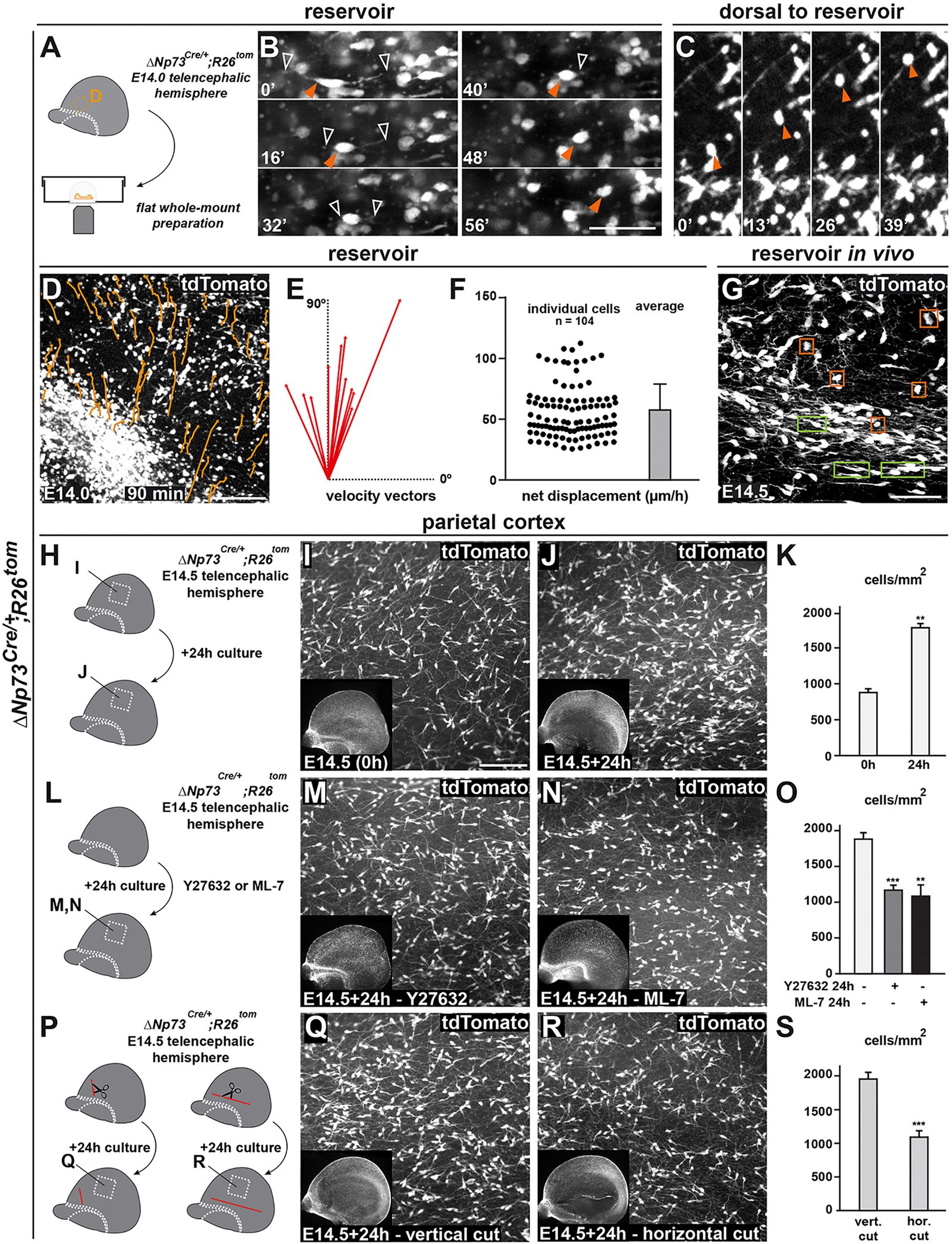
The neocortex undergoes extensive developmental growth, but how its architecture adapts to expansion remains largely unknown. Here, we investigated how early born Cajal-Retzius (CR) neurons, which regulate the assembly of cortical circuits, maintain a dense superficial distribution in the growing neocortex. We found that CR cell density is sustained by an activity-dependent importation of olfactory CR cells, which migrate into the neocortex after they have acted as axonal guidepost cells in the olfactory system. Furthermore, using mouse genetics, we showed that CR cell density severely affects the architecture of layer 1, a key site of input integration for neocortical networks, leading to an excitation/inhibition ratio imbalance. Our study reveals that neurons reenter migration several days after their initial positioning, thereby performing sequential developmental roles in olfactory cortex and neocortex. This atypical process is essential to regulate CR cell density during growth, which in turn ensures the correct wiring of neocortical circuitry. VIDEO ABSTRACT.
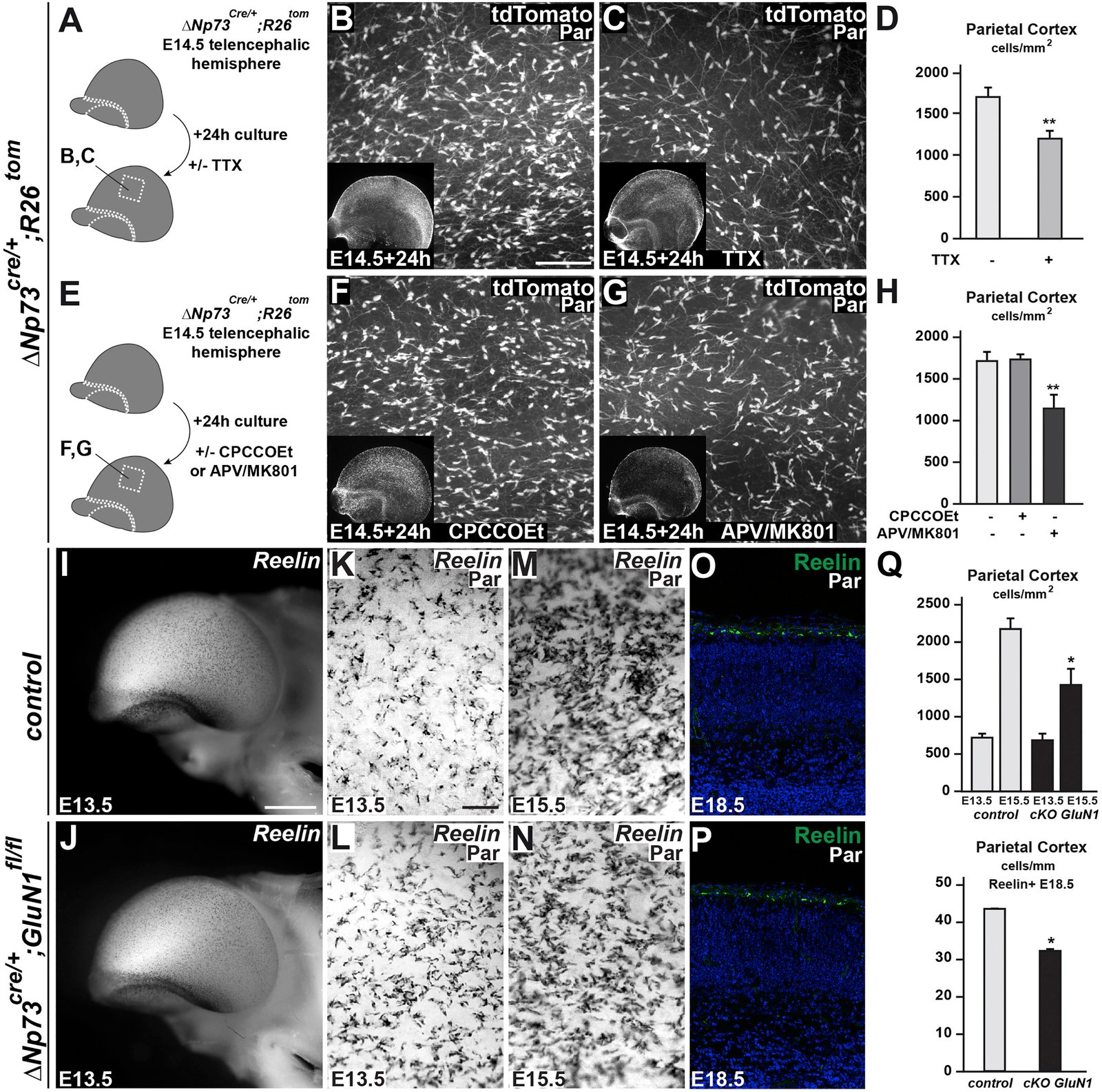
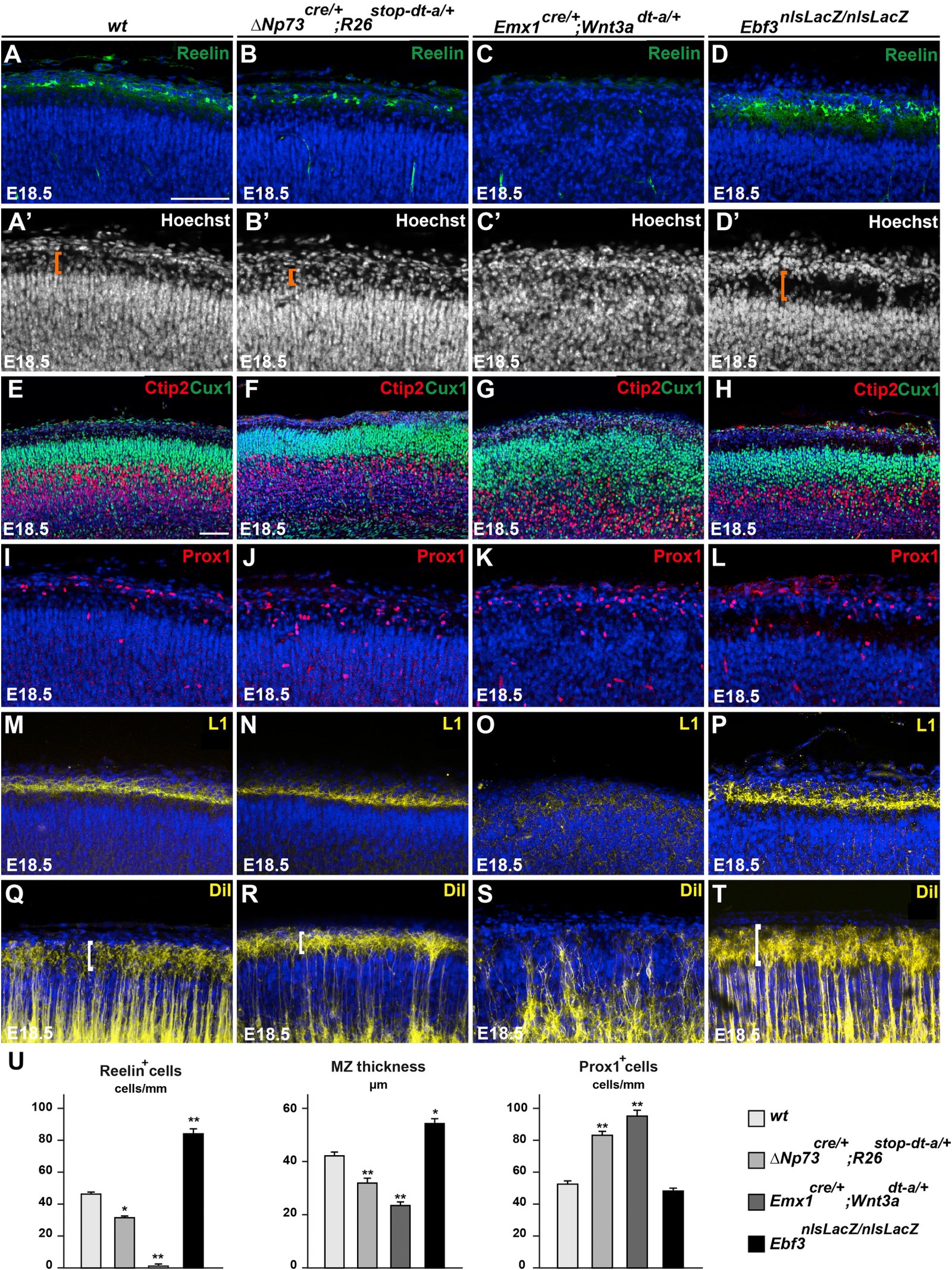
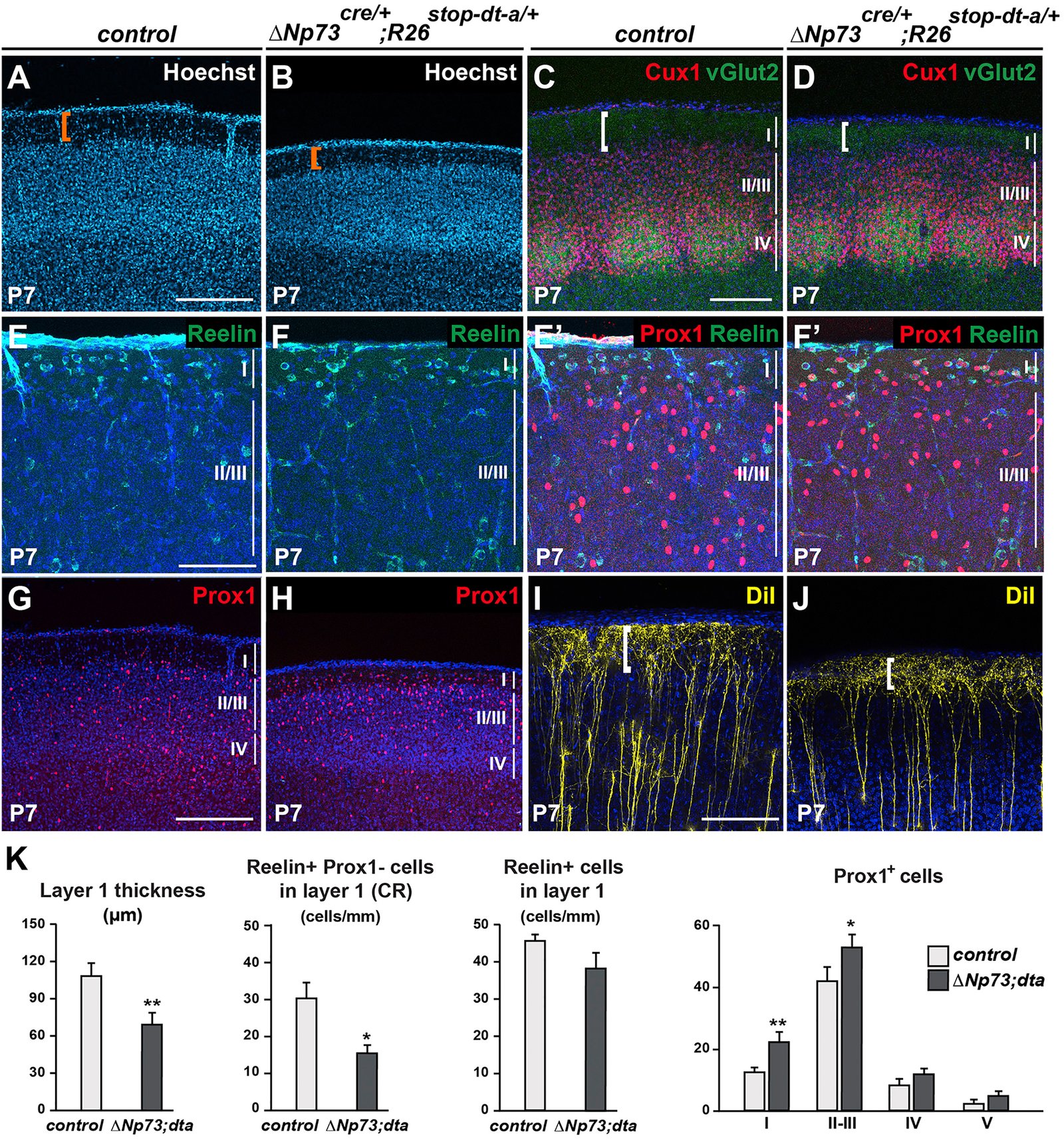
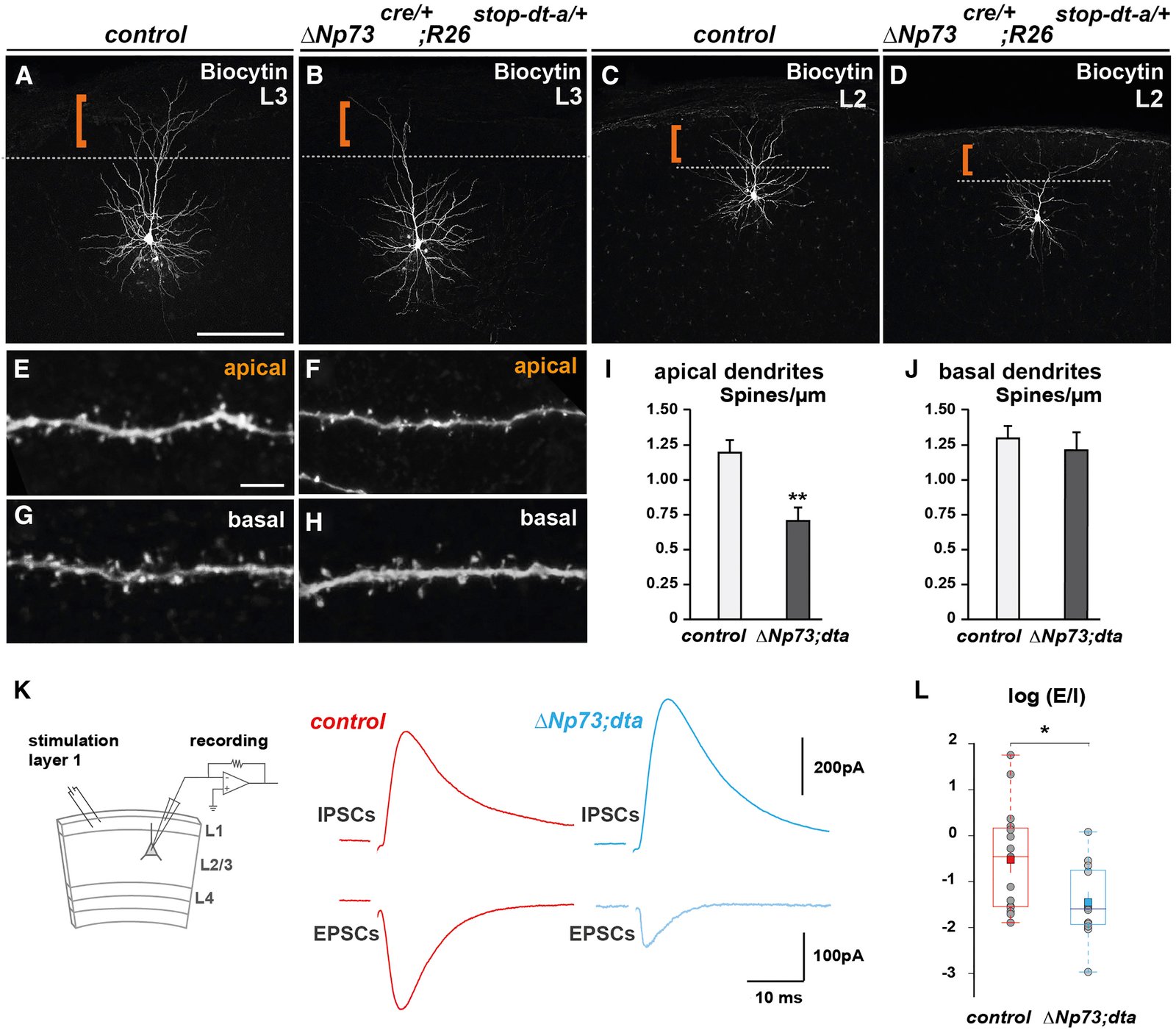
March 2016
Numerous studies have shown that cerebellar function is related to the plasticity at the synapses between parallel fibers and Purkinje cells. How specific input patterns determine plasticity outcomes, as well as the biophysics underlying plasticity of these synapses, remain unclear. Here, we characterize the patterns of activity that lead to postsynaptically expressed LTP using both in vivo and in vitro experiments. Similar to the requirements of LTD, we find that high-frequency bursts are necessary to trigger LTP and that this burst-dependent plasticity depends on presynaptic NMDA receptors and nitric oxide (NO) signaling. We provide direct evidence for calcium entry through presynaptic NMDA receptors in a subpopulation of parallel fiber varicosities. Finally, we develop and experimentally verify a mechanistic plasticity model based on NO and calcium signaling. The model reproduces plasticity outcomes from data and predicts the effect of arbitrary patterns of synaptic inputs on Purkinje cells, thereby providing a unified description of plasticity.
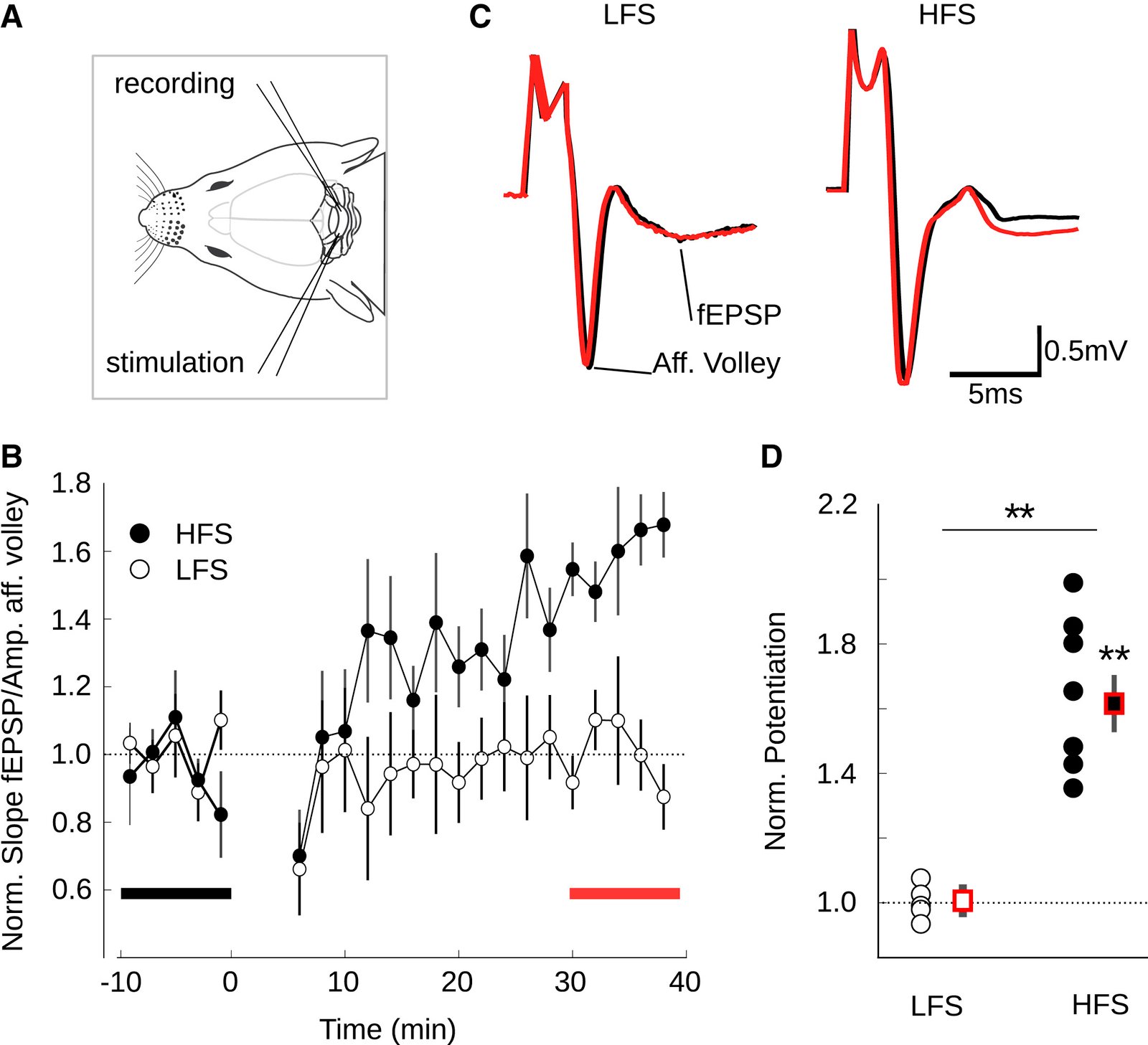
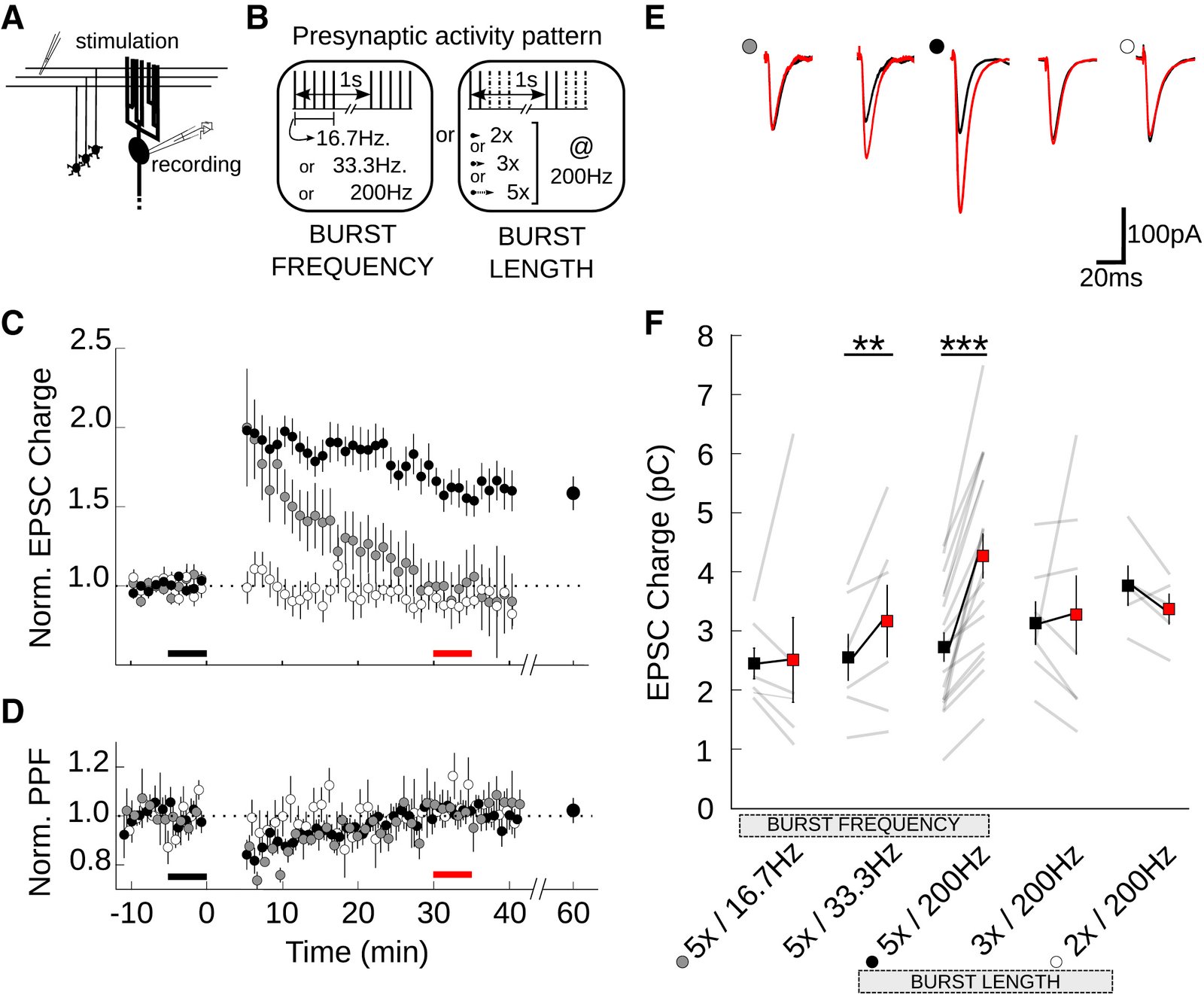
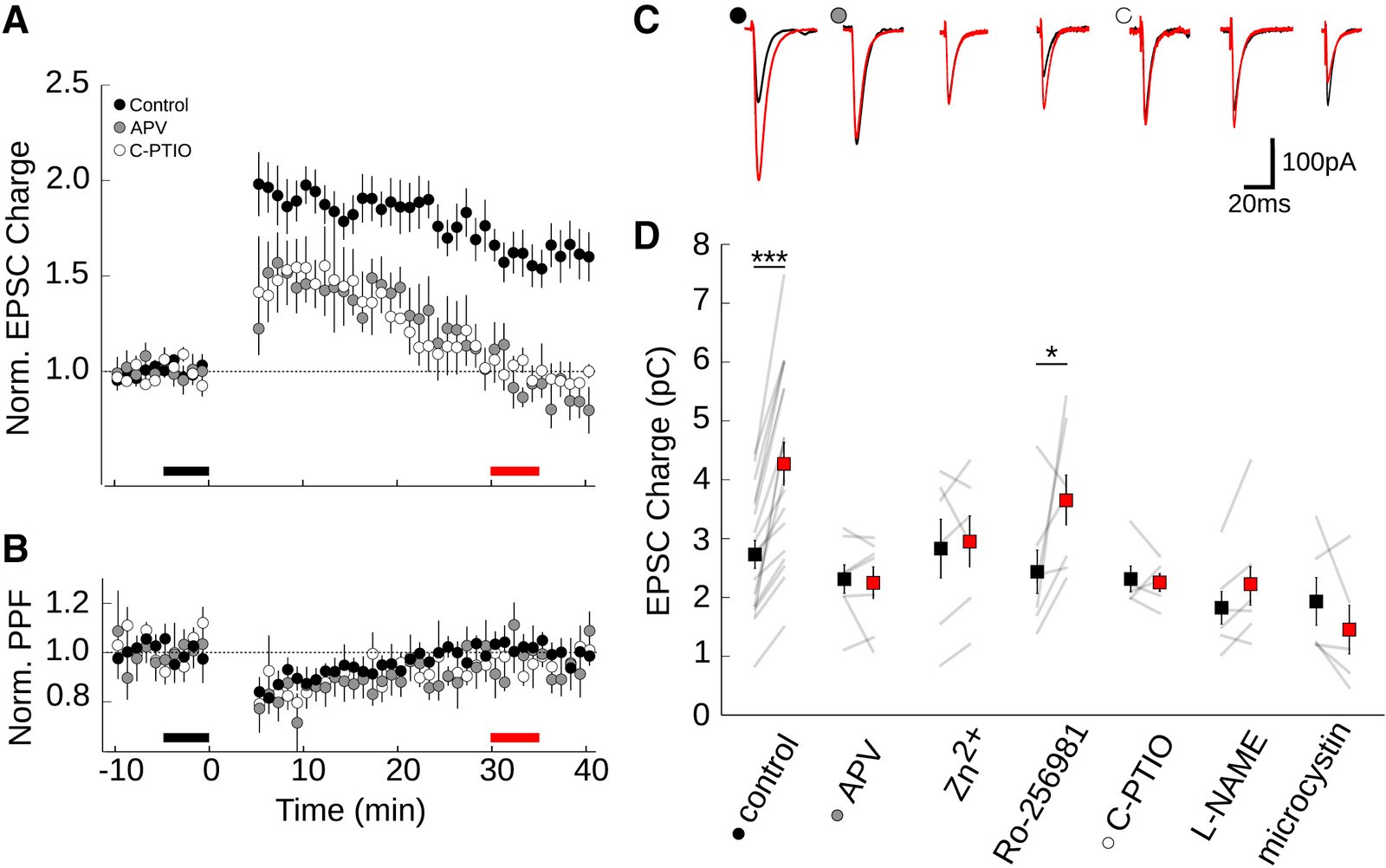
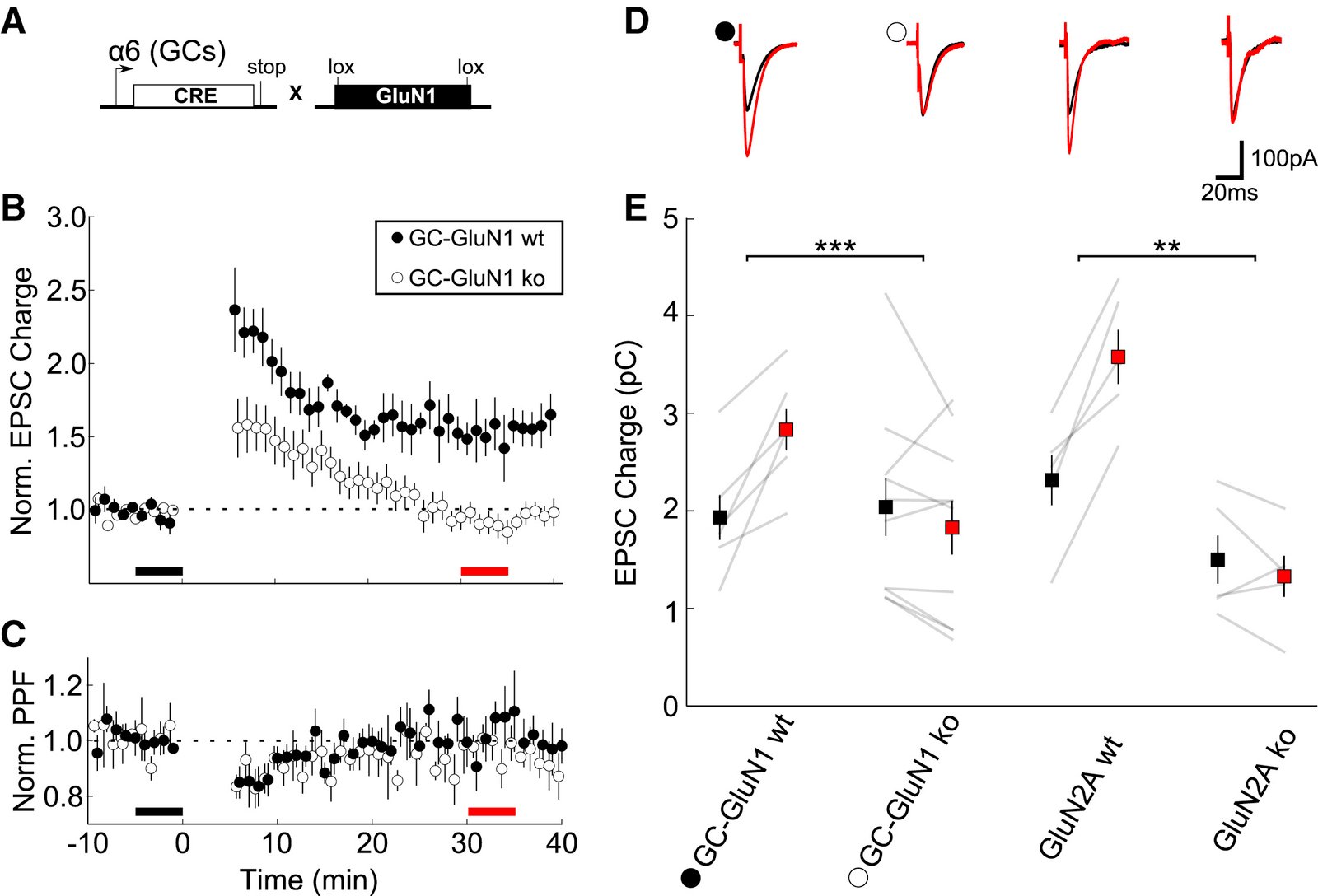
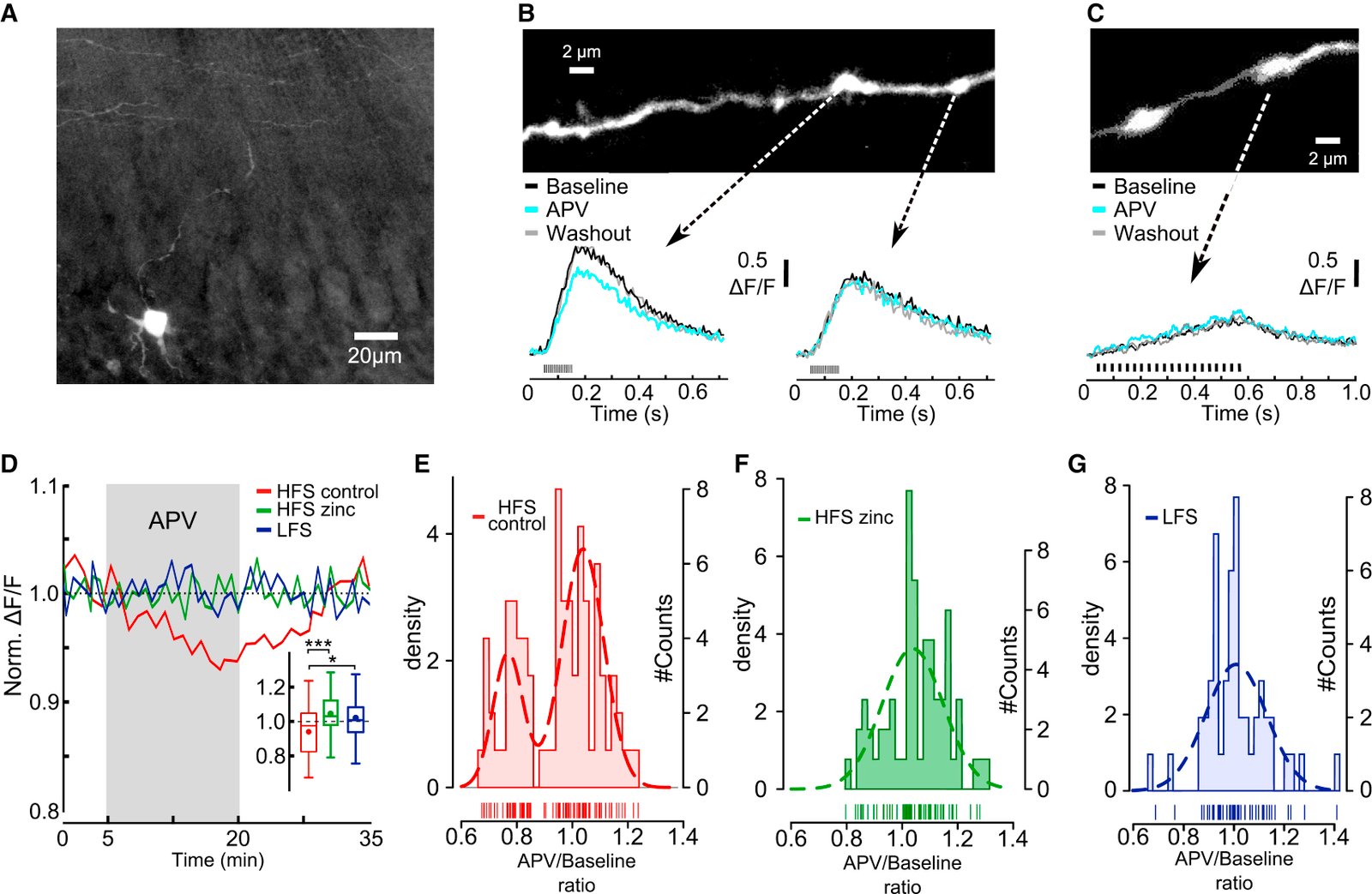
February 2016
In the cerebellum, sensory information is conveyed to Purkinje cells (PC) via the granule cell/parallel fibre (PF) pathway. Plasticity at the PF-PC synapse is considered to be a mechanism of information storage in motor learning. The induction of synaptic plasticity in the cerebellum and elsewhere usually involves intracellular Ca(2+) signals. Unusually, postsynaptic Ca(2+) signalling in PF-PC spines does not involve ionotropic glutamatergic receptors because postsynaptic NMDA receptors are absent and the AMPA receptors are Ca(2+) -impermeable; postsynaptic voltage-gated Ca(2+) channels therefore constitute the sole rapid Ca(2+) signalling mechanism. Low-threshold activated T-type calcium channels are present at the synapse, although their contribution to PF-PC synaptic responses is unknown. Taking advantage of 3,5-dichloro-N-[1-(2,2-dimethyl-tetrahydro-pyran-4-ylmethyl)-4-fluoro-piperidin-4-ylmethyl]-benzamide, a selective T-type channel antagonist, we show in the mouse that inhibition of these channels reduces PF-PC excitatory postsynaptic currents and excitatory postsynaptic potentials by 15-20%. This contribution was preserved during sparse input and repetitive activity. We characterized the biophysical properties of native T-type channels in young animals and modelled their activation during simulated dendritic excitatory postsynaptic potential waveforms. The comparison of modelled and observed synaptic responses suggests that T-type channels only activate in spines that are strongly depolarized by their synaptic input, a process requiring a high spine neck resistance. This brief and local activation ensures that T-type channels rapidly deactivate, thereby limiting inactivation during repetitive synaptic activity. T-type channels are therefore ideally situated to provide synaptic Ca(2+) entry at PF-PC spines.
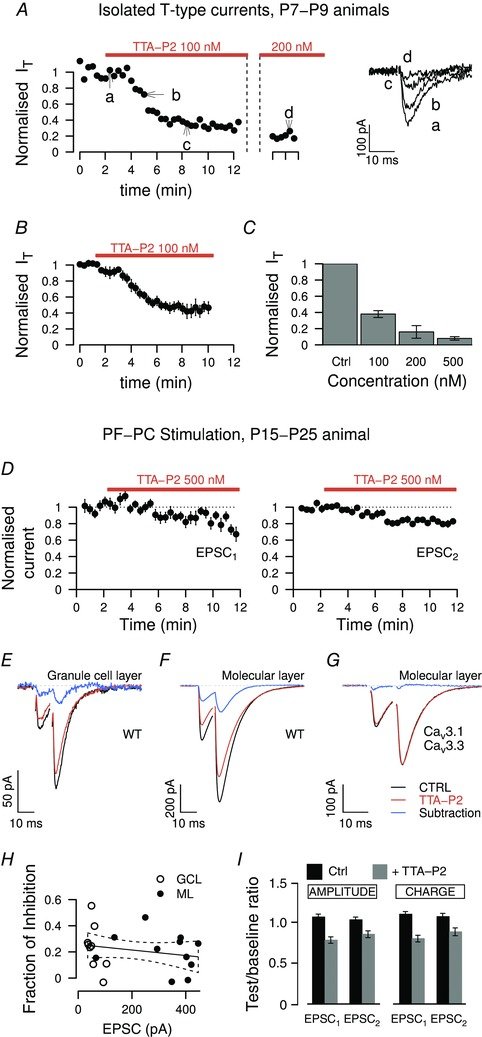
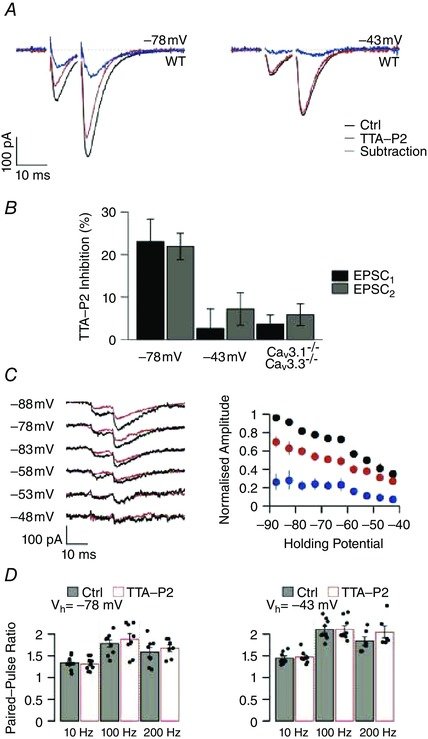
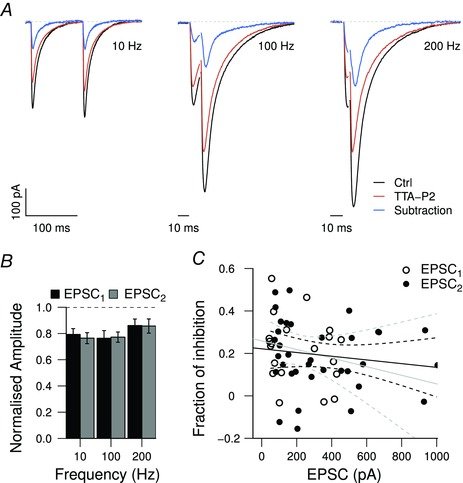
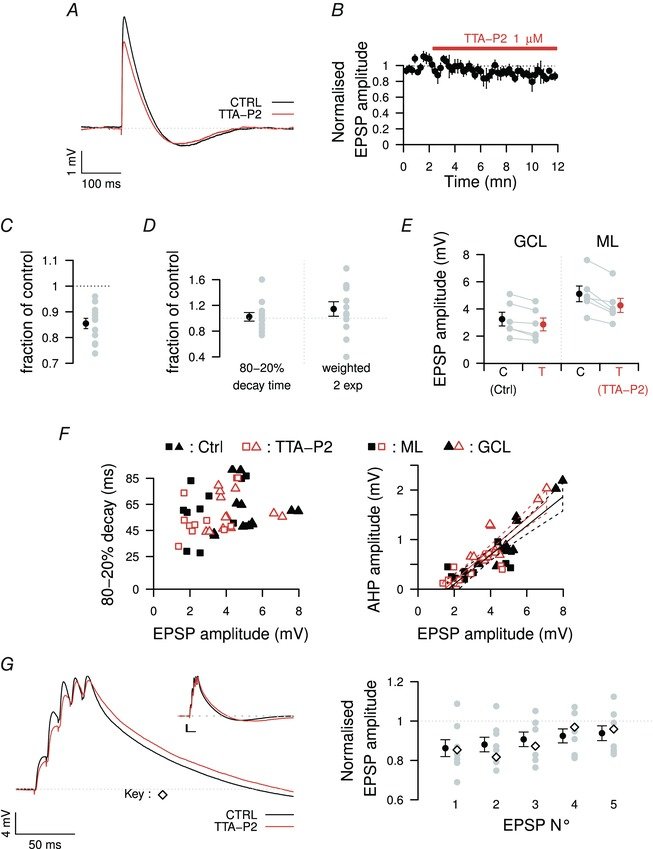
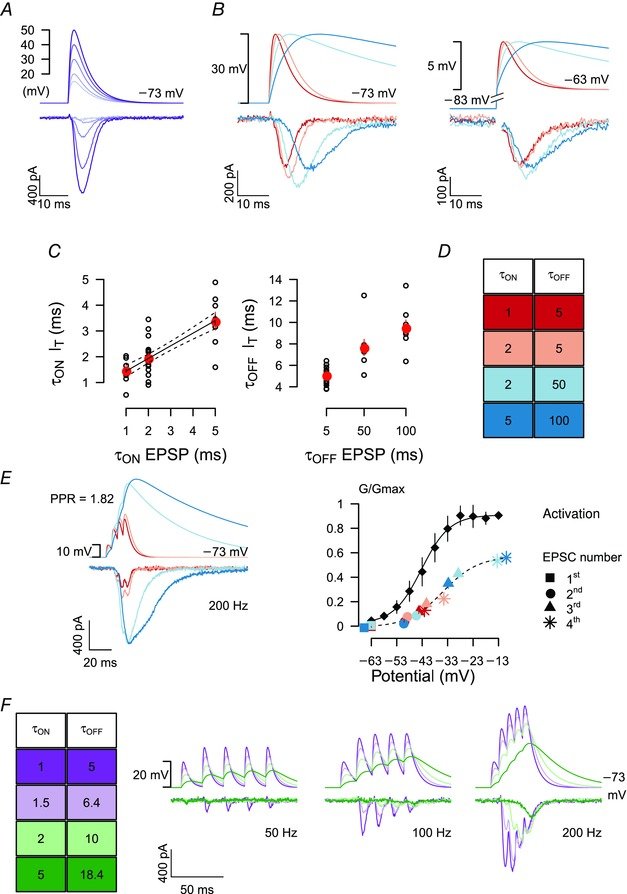
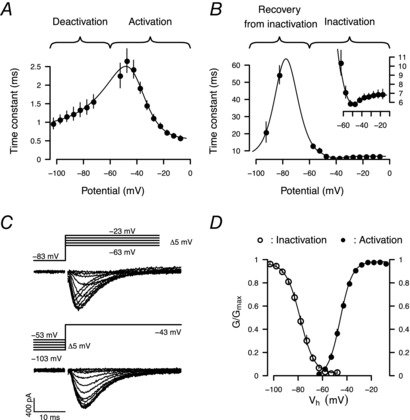
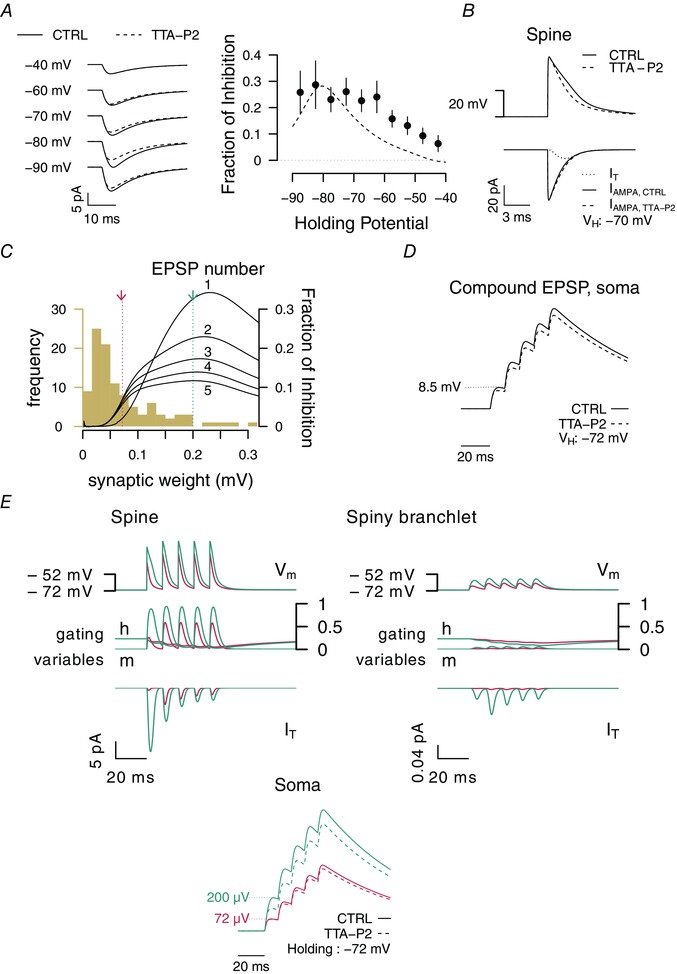
August 2015
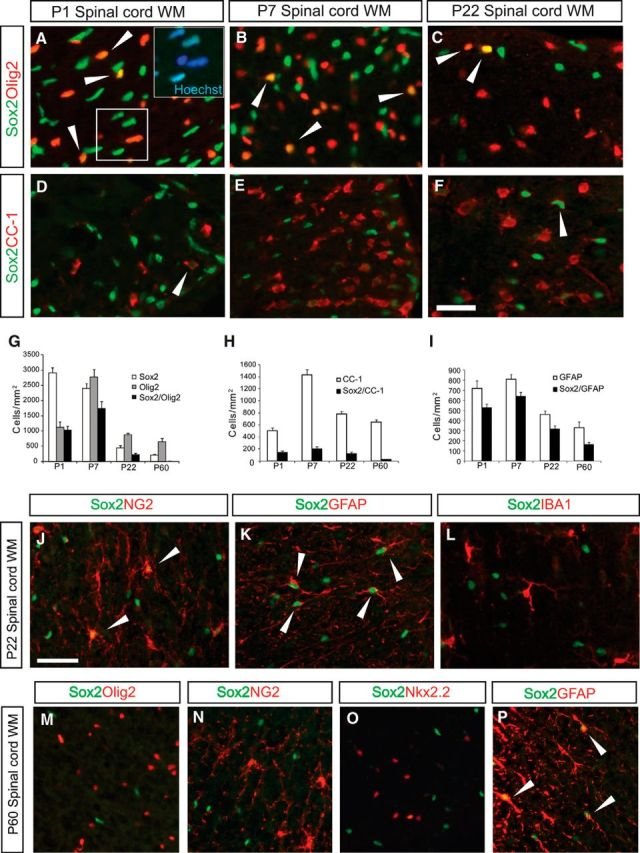
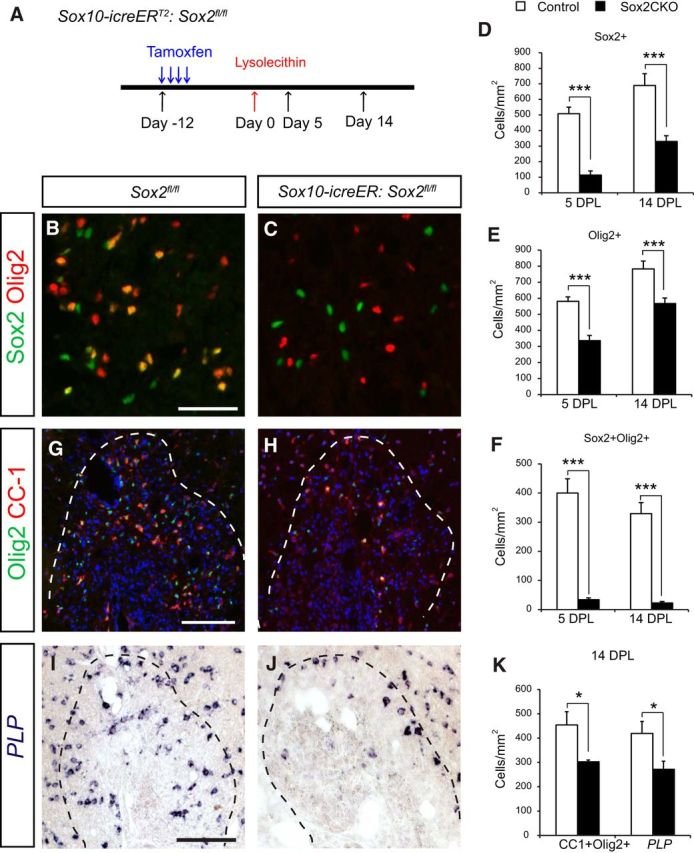
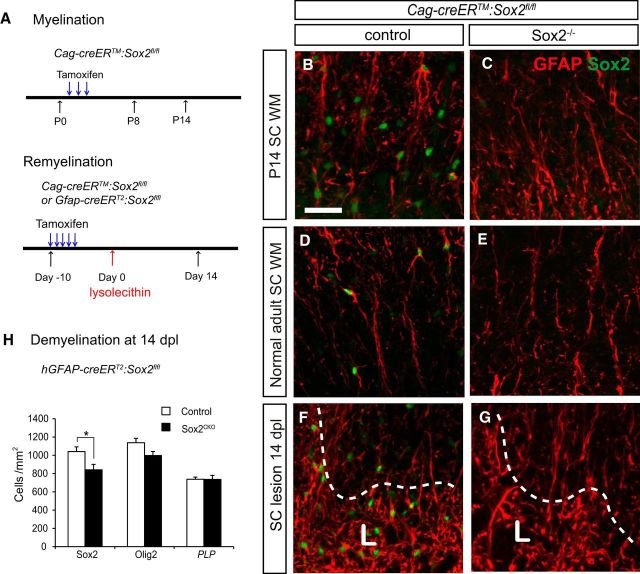
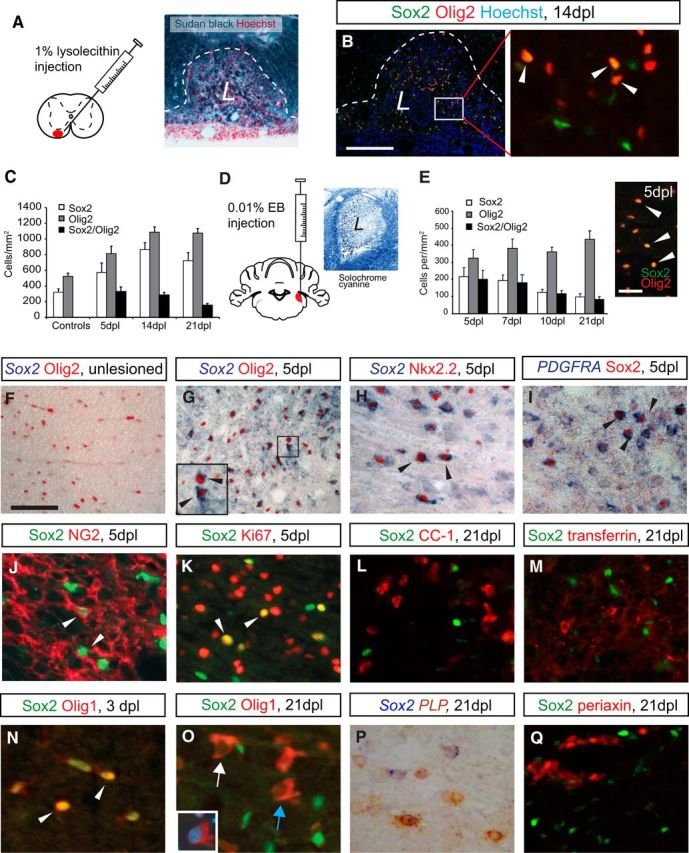
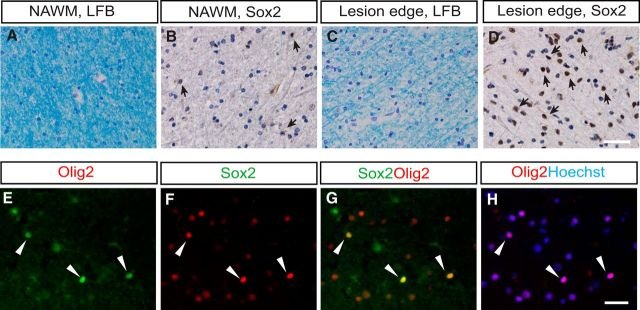
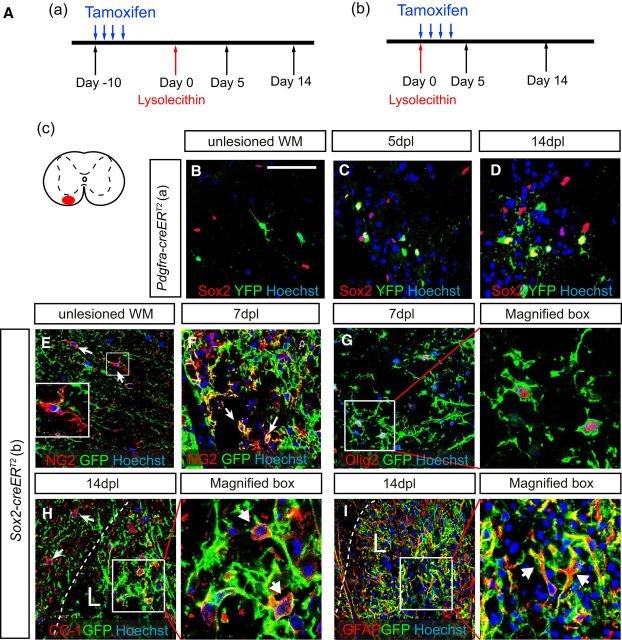
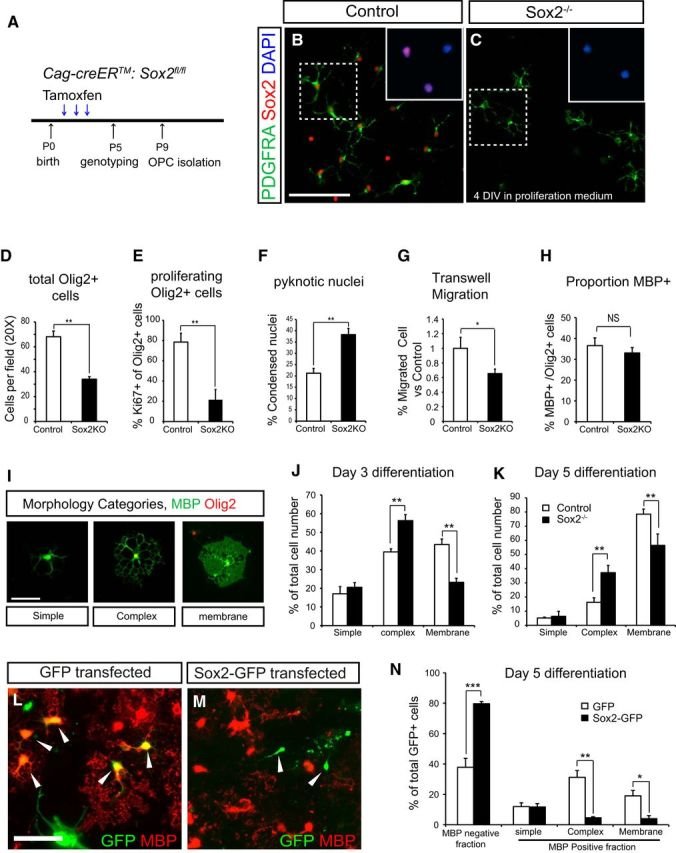
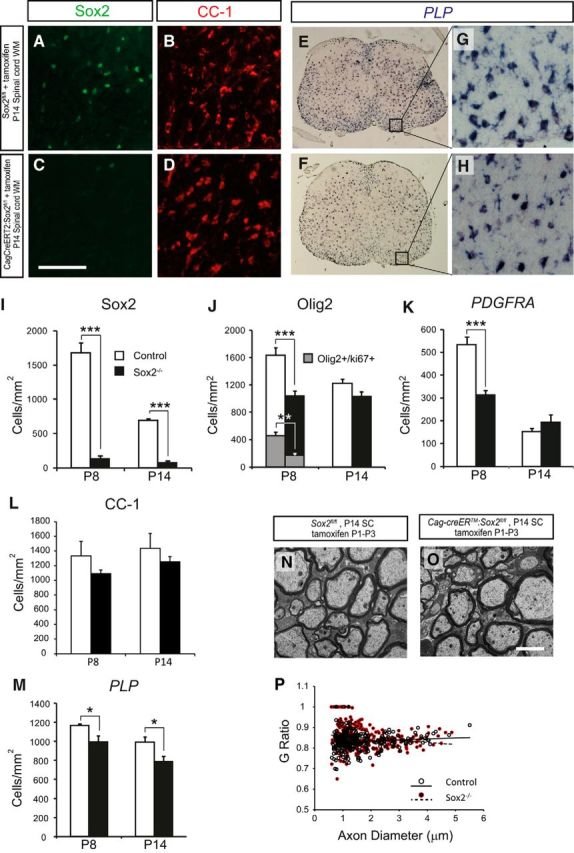
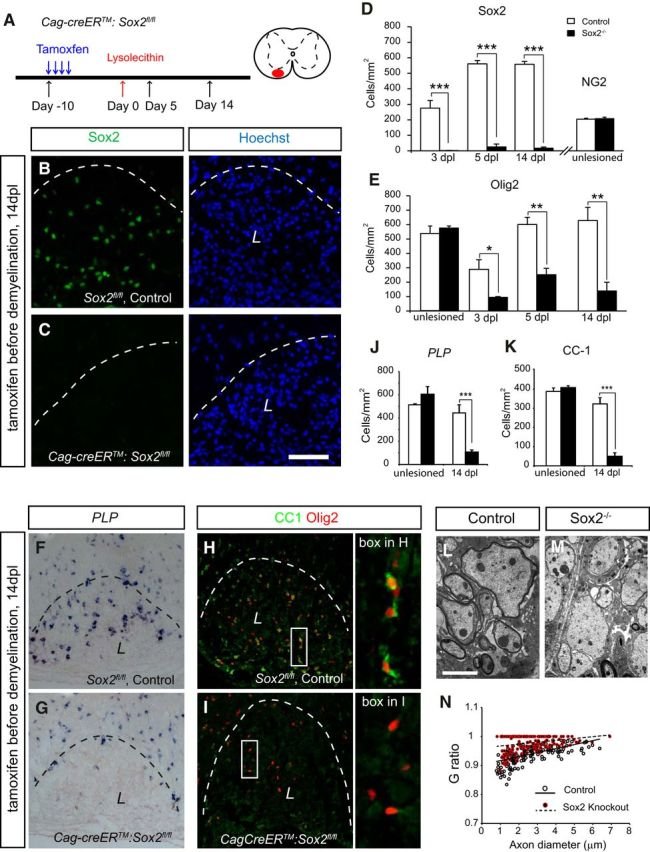
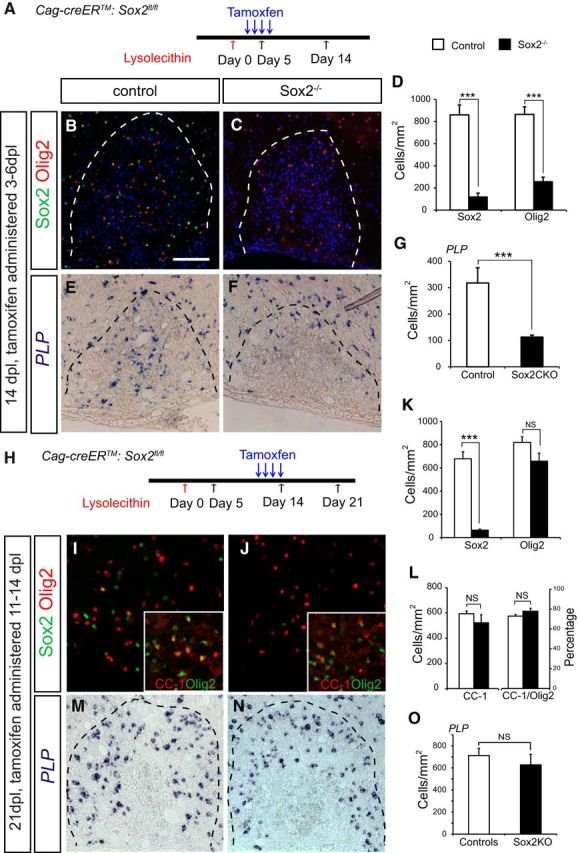
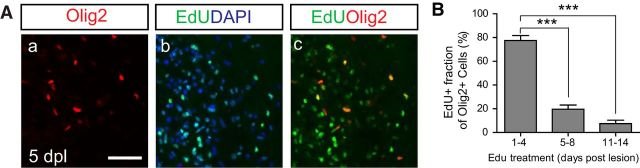
The Sox family of transcription factors have been widely studied in the context of oligodendrocyte development. However, comparatively little is known about the role of Sox2, especially during CNS remyelination. Here we show that the expression of Sox2 occurs in oligodendrocyte progenitor cells (OPCs) in rodent models during myelination and in activated adult OPCs responding to demyelination, and is also detected in multiple sclerosis lesions. In normal adult white matter of both mice and rats, it is neither expressed by adult OPCs nor by oligodendrocytes (although it is expressed by a subpopulation of adult astrocytes). Overexpression of Sox2 in rat OPCs in vitro maintains the cells in a proliferative state and inhibits differentiation, while Sox2 knockout results in decreased OPC proliferation and survival, suggesting that Sox2 contributes to the expansion of OPCs during the recruitment phase of remyelination. Loss of function in cultured mouse OPCs also results in an impaired ability to undergo normal differentiation in response to differentiation signals, suggesting that Sox2 expression in activated OPCs also primes these cells to eventually undergo differentiation. In vivo studies on remyelination following experimental toxin-induced demyelination in mice with inducible loss of Sox2 revealed impaired remyelination, which was largely due to a profound attenuation of OPC recruitment and likely also due to impaired differentiation. Our results reveal a key role of Sox2 expression in OPCs responding to demyelination, enabling them to effectively contribute to remyelination.
February 2015
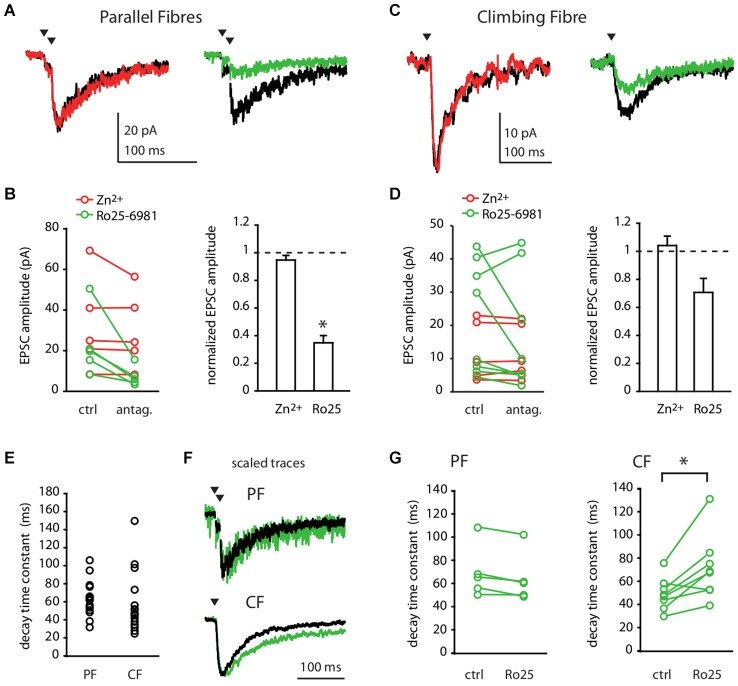
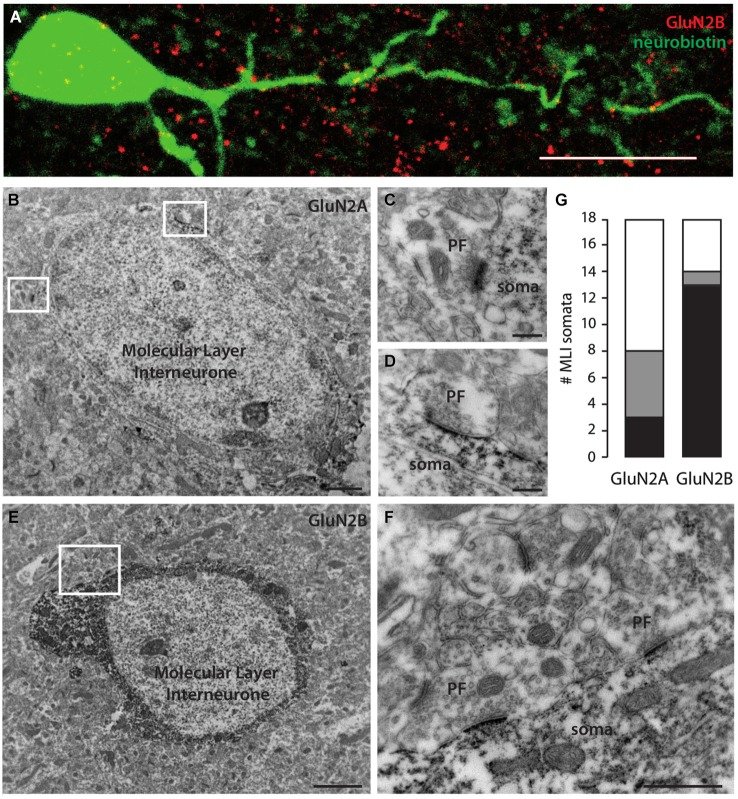
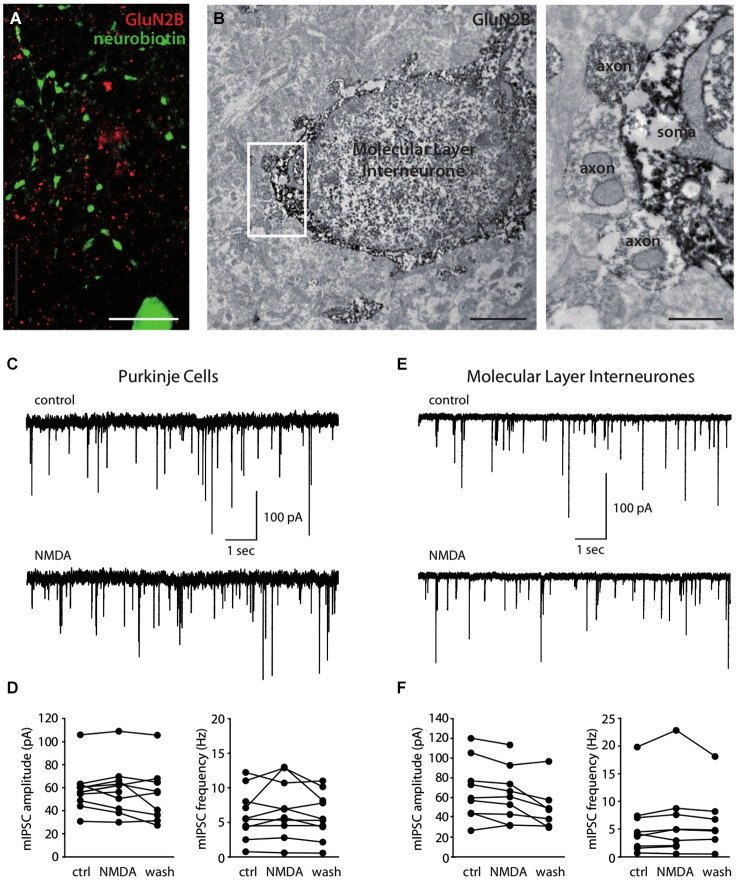
N-methyl-D-aspartate receptors (NMDARs) in cerebellar molecular layer interneurons (MLIs) are expressed and activated in unusual ways: at parallel fibre (PF) synapses they are only recruited by repetitive stimuli, suggesting an extrasynaptic location, whereas their activation by climbing fibre is purely mediated by spillover. NMDARs are thought to play an important role in plasticity at different levels of the cerebellar circuitry. Evaluation of the location, functional properties and physiological roles of NMDARs will be facilitated by knowledge of the NMDAR isoforms recruited. Here we show that MLI-NMDARs activated by both PF and climbing fibre inputs have similar kinetics and contain GluN2B but not GluN2A subunits. On the other hand, no evidence was found of functional NMDARs in the axons of MLIs. At the PF-Purkinje cell (PF-PC) synapse, the activation of GluN2A-containing NMDARs has been shown to be necessary for the induction of long-term depression (LTD). Our results therefore provide a clear distinction between the NMDARs located on MLIs and those involved in plasticity at PF-PC synapses.
December 2013
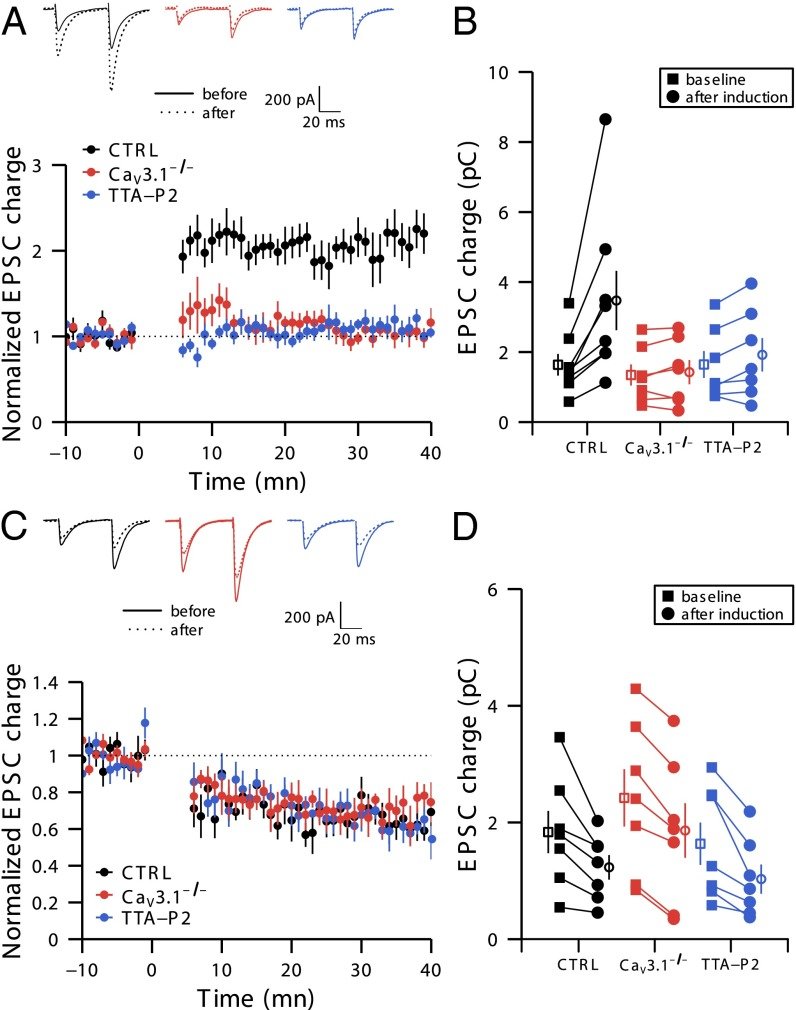
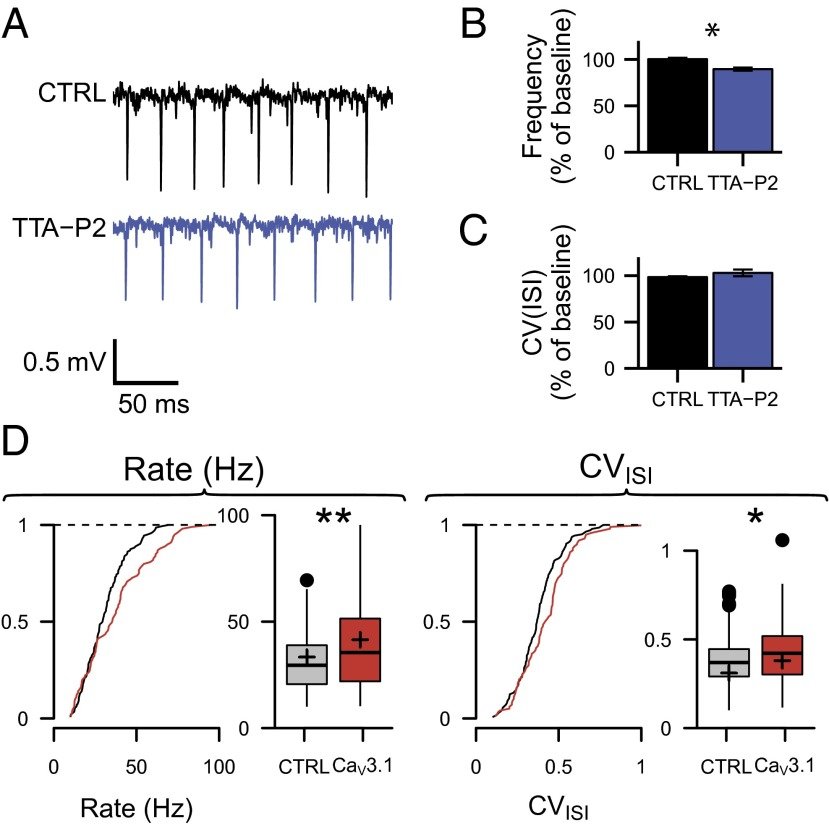
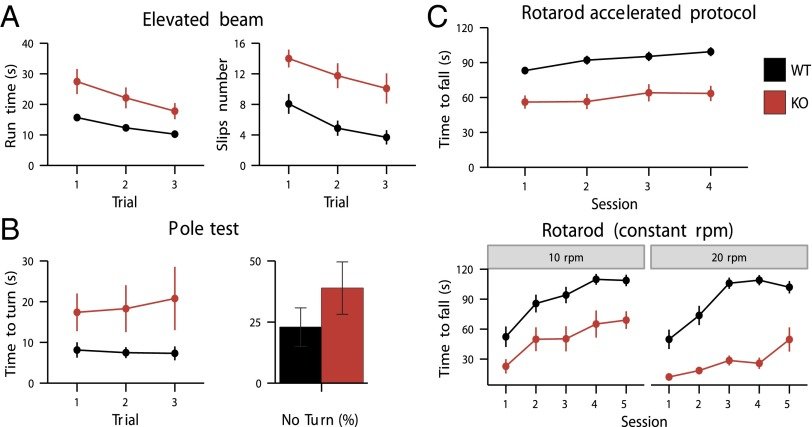
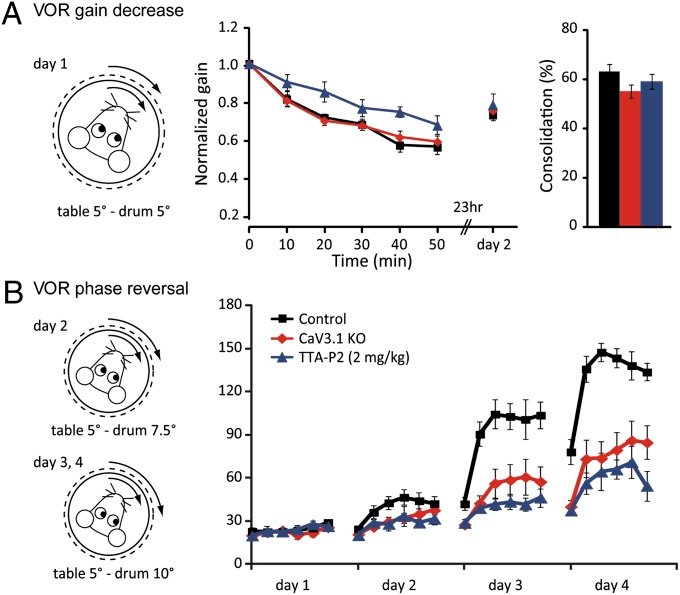
CaV3.1 T-type channels are abundant at the cerebellar synapse between parallel fibers and Purkinje cells where they contribute to synaptic depolarization. So far, no specific physiological function has been attributed to these channels neither as charge carriers nor more specifically as Ca(2+) carriers. Here we analyze their incidence on synaptic plasticity, motor behavior, and cerebellar motor learning, comparing WT animals and mice where T-type channel function has been abolished either by gene deletion or by acute pharmacological blockade. At the cellular level, we show that CaV3.1 channels are required for long-term potentiation at parallel fiber-Purkinje cell synapses. Moreover, basal simple spike discharge of the Purkinje cell in KO mice is modified. Acute or chronic T-type current blockade results in impaired motor performance in particular when a good body balance is required. Because motor behavior integrates reflexes and past memories of learned behavior, this suggests impaired learning. Indeed, subjecting the KO mice to a vestibulo-ocular reflex phase reversal test reveals impaired cerebellum-dependent motor learning. These data identify a role of low-voltage activated calcium channels in synaptic plasticity and establish a role for CaV3.1 channels in cerebellar learning.
July 2013
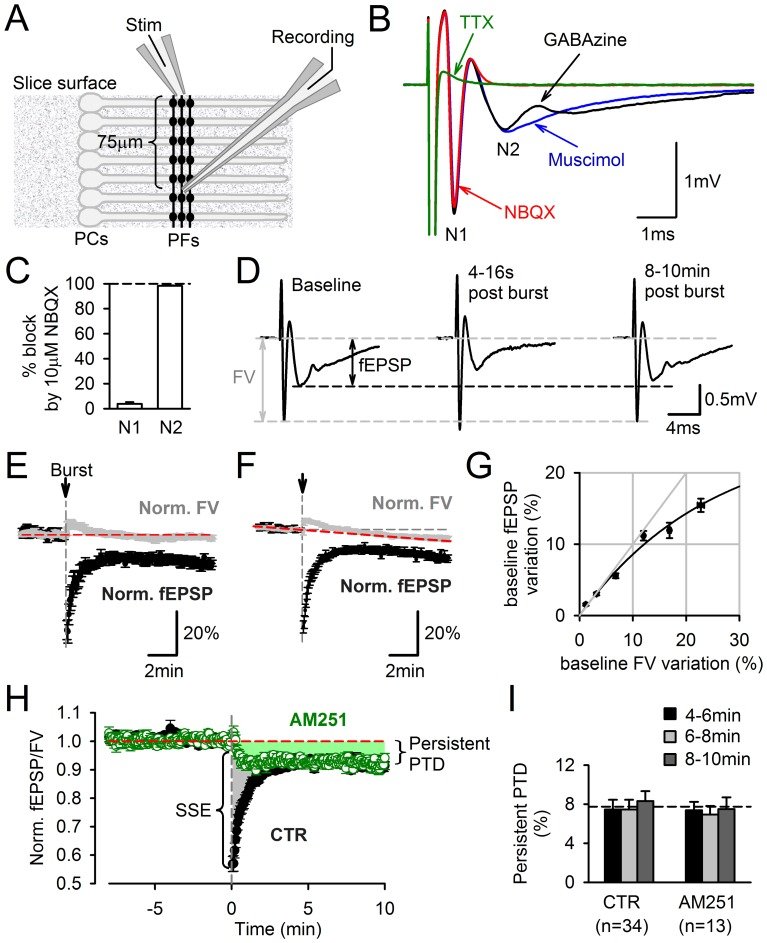
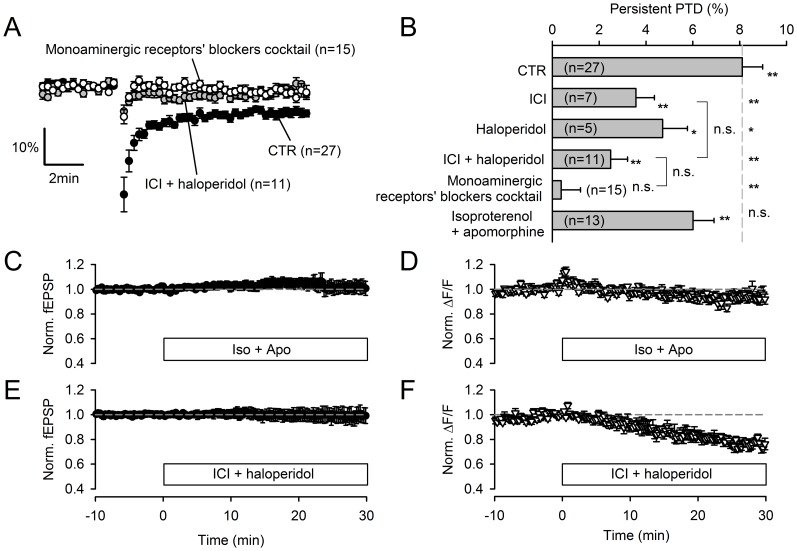
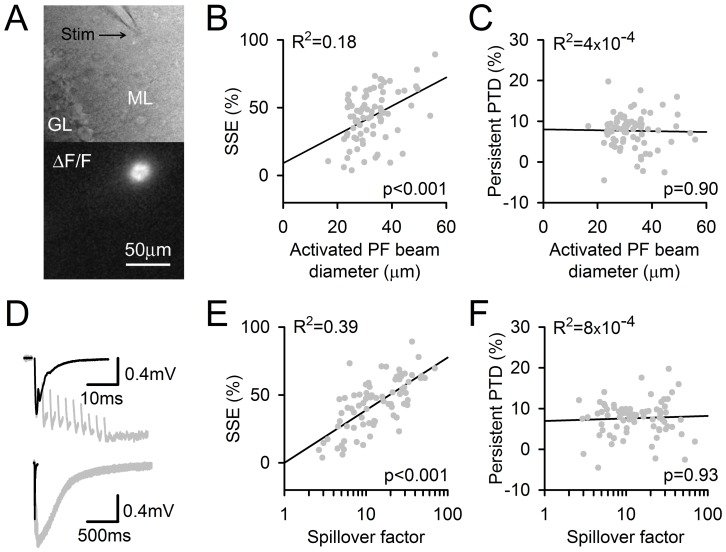
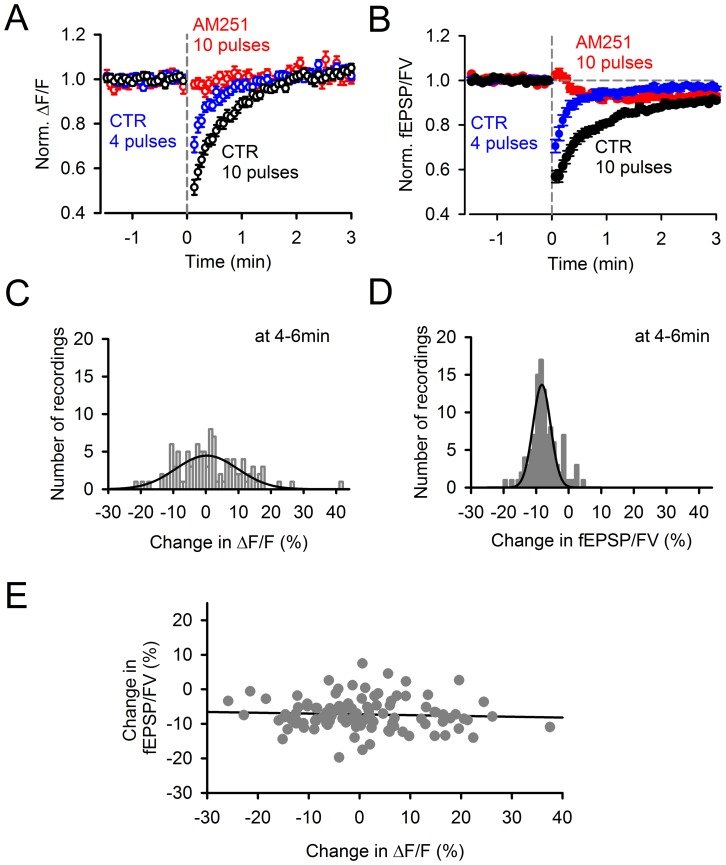
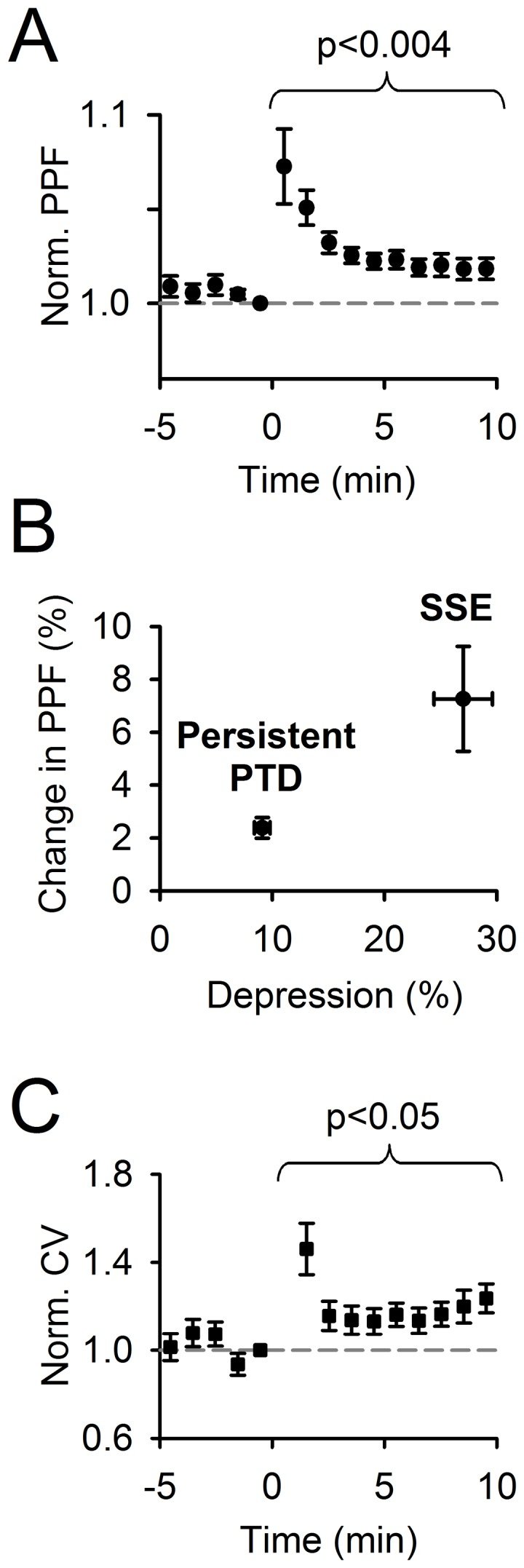
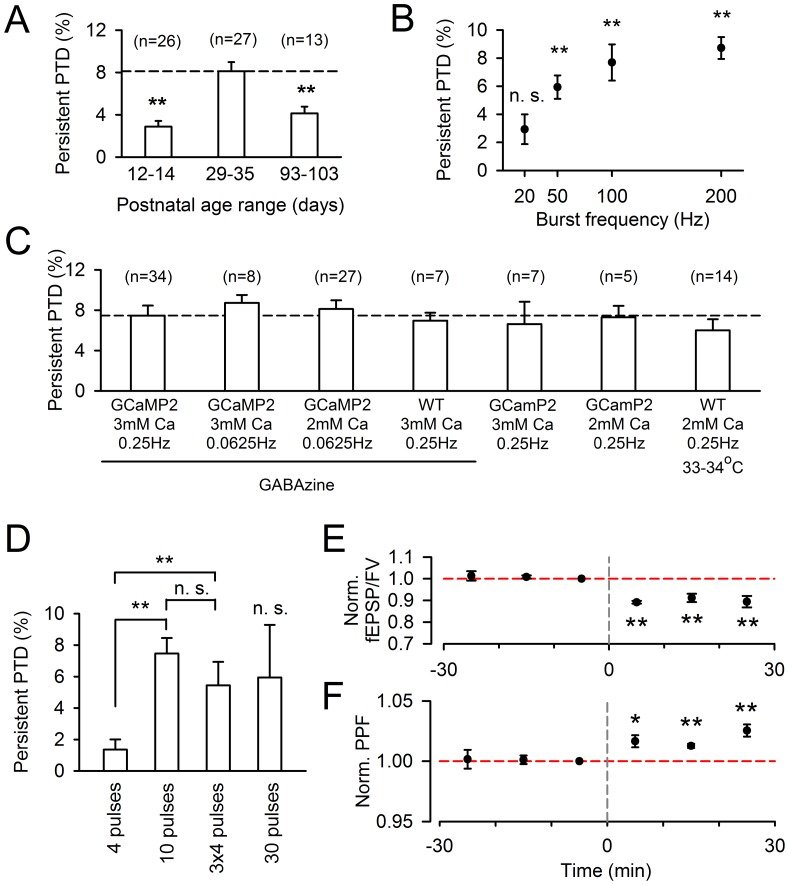
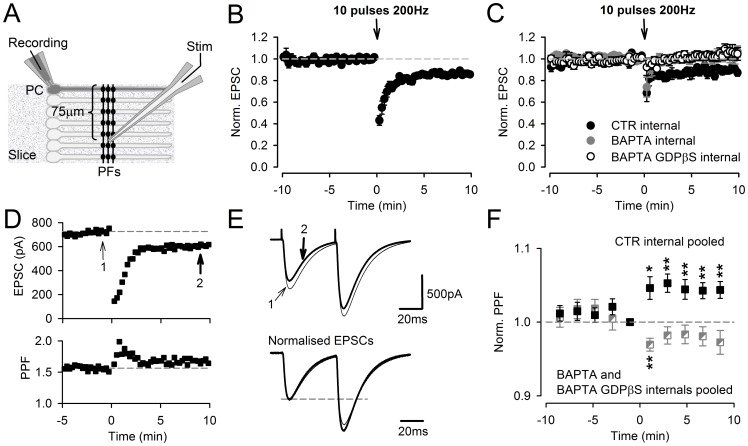
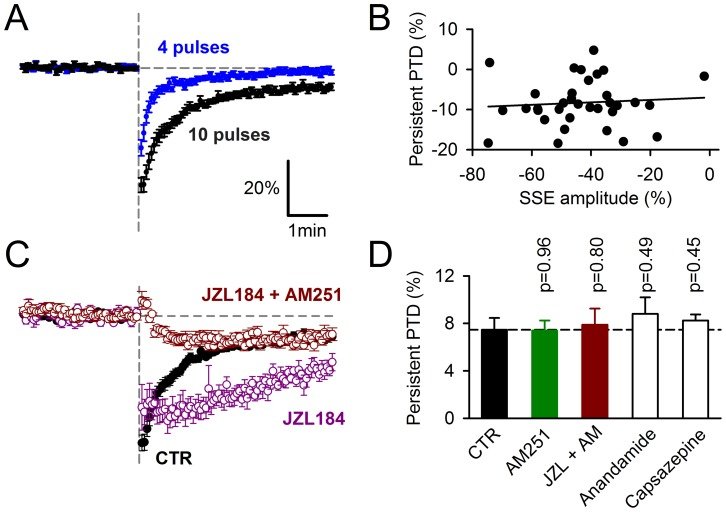
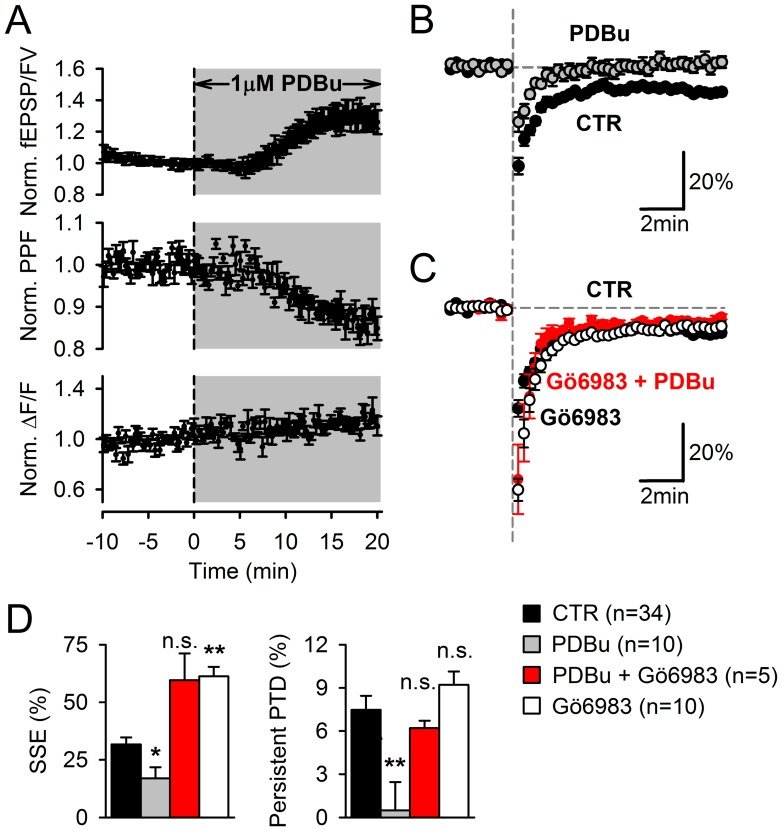
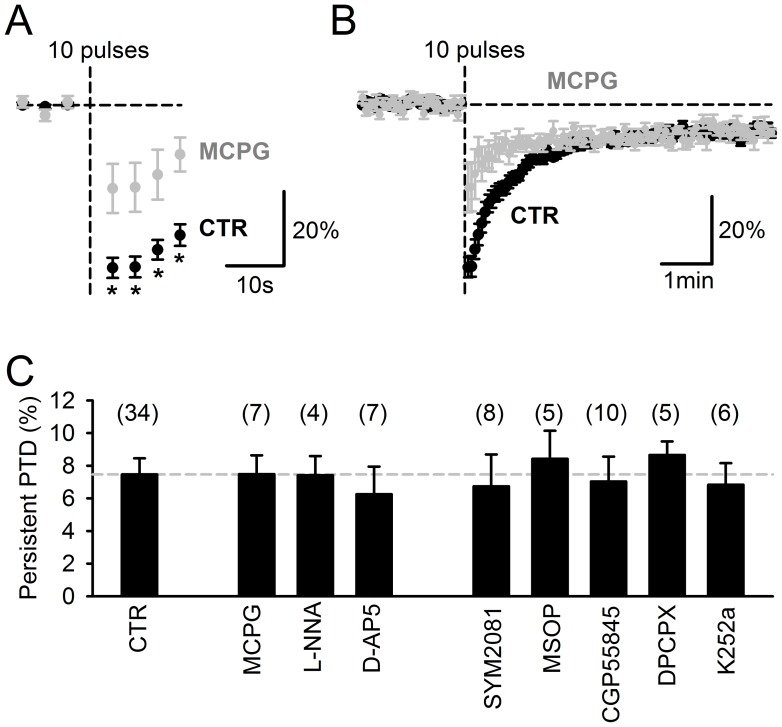
Plasticity at the cerebellar parallel fiber to Purkinje cell synapse may underlie information processing and motor learning. In vivo, parallel fibers appear to fire in short high frequency bursts likely to activate sparsely distributed synapses over the Purkinje cell dendritic tree. Here, we report that short parallel fiber tetanic stimulation evokes a ∼7-15% depression which develops over 2 min and lasts for at least 20 min. In contrast to the concomitantly evoked short-term endocannabinoid-mediated depression, this persistent posttetanic depression (PTD) does not exhibit a dependency on the spatial pattern of synapse activation and is not caused by any detectable change in presynaptic calcium signaling. This persistent PTD is however associated with increased paired-pulse facilitation and coefficient of variation of synaptic responses, suggesting that its expression is presynaptic. The chelation of postsynaptic calcium prevents its induction, suggesting that post- to presynaptic (retrograde) signaling is required. We rule out endocannabinoid signaling since the inhibition of type 1 cannabinoid receptors, monoacylglycerol lipase or vanilloid receptor 1, or incubation with anandamide had no detectable effect. The persistent PTD is maximal in pre-adolescent mice, abolished by adrenergic and dopaminergic receptors block, but unaffected by adrenergic and dopaminergic agonists. Our data unveils a novel form of plasticity at parallel fiber synapses: a persistent PTD induced by physiologically relevant input patterns, age-dependent, and strongly modulated by the monoaminergic system. We further provide evidence supporting that the plasticity mechanism involves retrograde signaling and presynaptic diacylglycerol.
Reviews
Current opinion in neurobiology
January 2018
In the classical view, postsynaptic NMDA receptors (NMDARs) trigger Hebbian plasticity via Ca2+ influx. However, unconventional presynaptic NMDARs (preNMDARs) which regulate both long-term and short-term plasticity at several synapse types have also been found. A lack of sufficiently specific experimental manipulations and a poor understanding of how preNMDARs signal have contributed to long-standing controversy surrounding these receptors. Although several prior studies linked preNMDARs to neocortical timing-dependent long-term depression (tLTD), a recent study argues that the NMDARs are actually postsynaptic and signal metabotropically, that is, without Ca2+. Other recent work indicates that, whereas ionotropic preNMDARs signaling controls evoked release, spontaneous release is regulated by metabotropic NMDAR signaling. We argue that elucidating unconventional NMDAR signaling modes-both presynaptically and metabotropically-is key to resolving the preNMDAR debate.
Neuroscience
December 2015
NMDA receptors (NMDARs) are glutamate-gated ion channels widely expressed in the central nervous system (CNS) and endowed with unique biophysical, pharmacological and signaling properties. These receptors are best known for their critical roles in synaptic plasticity and their implications in a variety of neurological and psychiatric disorders. Since their discovery three decades ago, NMDARs have been thoroughly studied as components of postsynaptic excitatory potentials. Early on, however, both anatomical and physiological evidence pointed out to the existence of NMDARs away from the postsynaptic density. Some were found to be extrasynaptic, while others seemed to be specifically present at presynaptic (i.e. axonal) elements. Although presynaptic NMDARs (preNMDARs) were at first thought to be exceptional, there is now strong evidence that these receptors are relatively widespread in the CNS and regulate synaptic strength in specific sets of synapses. In this review, we compile our current knowledge on preNMDARs, presenting their anatomical distribution, developmental regulation, subunit composition, activation mechanisms as well as their downstream effects on synapse function. Contentious issues that animate the field are also discussed. Finally, particular emphasis is put on the molecular and cellular diversity of preNMDARs which translates into a variety of effects, both short- and long-term, on synaptic efficacy. Overshadowed by their postsynaptic counterparts, preNMDARs are progressively emerging as important regulators of neuronal signaling.
